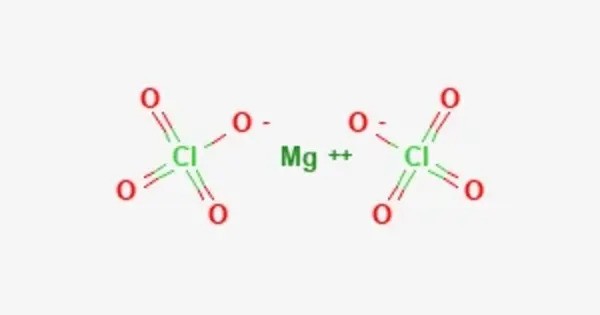
Magnesium perchlorate is a powerful oxidizing agent, with the formula Mg(ClO4)2. The salt is also a superior drying agent for gas analysis. It decomposes at 250 °C. The heat of formation is -568.90 kJ/mol. The enthalpy of solution is quite high, so reactions are done in large amounts of water to dilute it.
It is sold under the trade name anhydrone. Manufacture of this product on a semi-industrial scale was first performed by G. Frederick Smith in his garage in Urbana Illinois, but later at a permanent facility in Columbus, OH called G. Frederick Smith Chemical Co. He sold the magnesium perchlorate to A. H. Thomas Co., now Thomas Scientific, under the trade name Dehydrite.
Production
Magnesium perchlorate is produced by the reaction of magnesium hydroxide and perchloric acid. Magnesium perchlorate and other perchlorates have been found on Mars.
Properties
- Chemical formula: Mg(ClO4)2
- Molar mass: 223.206 g/mol
- Appearance: white powder, deliquescent
- Odor: odorless
- Density: 2.21 g/cm3 (anhydrous), 1.98 g/cm3 (hexahydrate)
- Melting point: 251 °C (484 °F; 524 K) (anhydrous), 95-100 °C (hexahydrate)
- Boiling point: decomposition
- Solubility in water: 99.3 g/100 mL
- Solubility in ethanol: 23.96 g/100 mL
Chemical Properties
- Oxidizing Agent: Magnesium perchlorate is a strong oxidizer due to the perchlorate ion (ClO₄⁻), which contains chlorine in its highest oxidation state (+7).
- Reactivity: Reacts violently with organic materials or reducing agents.
- Thermal Decomposition: Releases oxygen gas and forms magnesium chloride and other decomposition products.
Uses
It is used as desiccant to dry gas or air samples, but is no longer advised, for use as a general desiccant, due to hazards inherent in perchlorates. It is dried by heating at 220 °C under vacuum.
- Desiccant: It is widely used as a drying agent in laboratories (often in desiccators) because it is highly hygroscopic.
- Analytical Chemistry: Sometimes used in analytical methods for detecting water or moisture.
- Propellants/Explosives: Perchlorate compounds can be components in pyrotechnics, though magnesium perchlorate is less commonly used than others like ammonium perchlorate.
Safety & Hazards
- Oxidizer: Strong; it can enhance combustion of flammable materials.
- Toxicity: Perchlorates may disrupt thyroid function by interfering with iodine uptake.
- Handling: Should be used with gloves and eye protection; avoid contact with organic materials or heat.