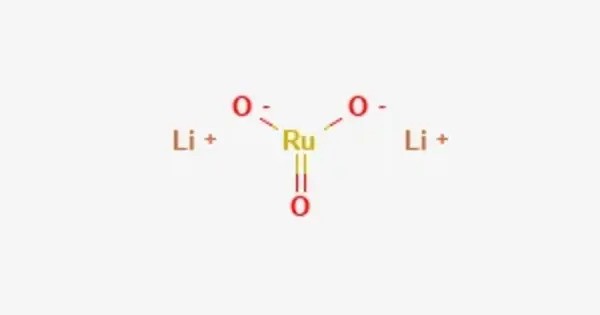
Lithium ruthenate (Li₂RuO₃) is a chemical compound composed of lithium, ruthenium, and oxygen, characterized by a layered honeycomb crystal structure. It has a layered honeycomb crystal structure, and can be prepared by direct calcination of Ru metal and lithium carbonate at ca. 700 °C.
It is synthesized through direct calcination of ruthenium metal and lithium carbonate at approximately 700°C. It is a potential lithium-ion battery electrode material, though this application is hindered by the high costs of Ru, as compared to the cheaper Li2MnO3 alternative. It undergoes structural transformations under aqueous conditions and exhibits partial Ru⁵⁺/Ru⁴⁺ redox activity during cycling, making it a candidate for advanced energy storage systems.
Properties
- Chemical formula: Li2RuO3
- Appearance: Dark blue crystals
Electrochemical Properties
- Li₂RuO₃ is a potential electrode material for lithium-ion batteries due to its ability to undergo reversible lithium insertion and extraction. However, its practical use is limited by the high cost of ruthenium compared to alternatives like Li₂MnO₃.
- In basic aqueous solutions, it undergoes structural transformation from an O3 to an O1 structure upon slow delithiation, losing approximately 1.25 Li ions per formula unit. This transformation is associated with partial exchange of Li₂O with H₂O.
- Its electrochemical performance is influenced by its unique structural features, electronic structure, stability, and transport properties, making it a candidate for advanced battery applications despite cost barriers.
Natural Occurrence
Lithium ruthenate does not occur naturally in significant quantities. Ruthenium, a key component, is a rare transition metal found as a minor component in platinum group metal ores, primarily in the Ural Mountains, North and South America, and in pentlandite deposits in Sudbury, Ontario, and pyroxenite deposits in South Africa.
The native form of ruthenium is a rare mineral, often with iridium substituting for ruthenium in its structure. Lithium, another component, is typically mined from pegmatitic minerals (e.g., spodumene, petalite, lepidolite) or subsurface brines, with major producers being Australia and Chile.
Synthetic Production
- Li₂RuO₃ is synthesized in the laboratory, typically by direct calcination of ruthenium metal and lithium carbonate at approximately 700 °C.
- Alternative methods include hydrothermal synthesis, which allows control over the dimensionality and topology of Ru ion arrangements. For example, NaCl-type lithium ruthenates like Li₂RuO₃ have been synthesized hydrothermally to study their magnetic properties.
- High-purity Li₂RuO₃ powder is produced in research quantities for applications such as battery electrode materials.
Applications
Li₂RuO₃ is primarily investigated for use as an electrode material in lithium-ion batteries due to its structural and electrochemical properties. Its metallic conductivity and layered structure make it promising for energy storage, though its commercial use is limited by the high cost of ruthenium.
Limitations
The high cost of ruthenium compared to alternatives like manganese-based compounds (e.g., Li₂MnO₃) hinders large-scale adoption in battery technology. Additionally, its reactivity with water under certain conditions requires careful handling in electrochemical applications.