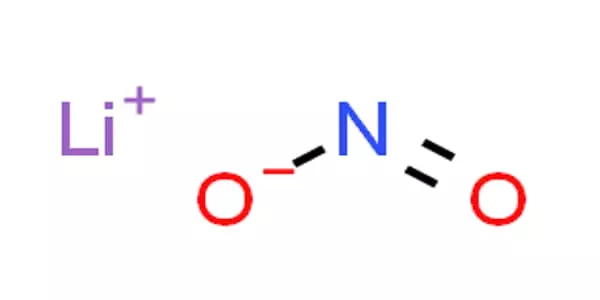
The lithium salt of nitrous acid, with the formula LiNO2, is lithium nitrite. This compound is highly hygroscopic and water-soluble. It is used in mortar as a corrosion inhibitor. It is one of the most rapid Li ionic conductors. Because of its ability to nitrosate ketones under certain conditions, it is also used in the production of explosives.
Lithium nitride is a model material for energy applications involving the transport of lithium ions because it is the only stable binary compound formed between an alkali metal and nitrogen.
Properties
It is a crystalline (sand-like) powder or granule that is colorless or white. This compound is highly hygroscopic and water-soluble. It is used in rocket propellants, pyrotechnics, and ceramics, as well as a laboratory reagent and heat exchange medium.
- Melting point: >100℃
- Boiling point: decomposes
- Density: 1.615
- Water Solubility: very soluble H2O
- Molecular Weight: 68.95
- Appearance: White
Preparation
Lithium nitrate (LiNO3) will undergo thermal decomposition above 500 °C to yield the evolution of lithium nitrite and oxygen as in the following reaction:
2LiNO3 → 2LiNO2 + O2 (at ~500 °C)
Lithium nitrite can also be prepared by the reaction of nitric oxide (NO) with lithium hydroxide (LiOH) as shown below:
4NO + 2LiOH → 2LiNO2 + N2O + H2O
6NO + 4LiOH → 4LiNO2 + N2 + 2H2O
Industrial uses
Corrosion is a common problem with reinforcement bars, ready-mixed concrete, and repair materials. Because of chloride attack and carbonatation, these resources will degrade rapidly. This not only shortens the service lives of such materials, but also necessitates a significant cost for the repair of such defects. In the construction industry, lithium nitrite and calcium nitrite are commonly used to protect reinforced concrete structures from corrosion. Unlike calcium nitrite inhibitors, lithium nitrite is highly valued for corrosion inhibition and carbonation resistance when an accelerated hardening process is not used and a high concentration of 10% or more cement by weight is added.
In general, destructive methods have been used to investigate the efficacy of such inhibitors. These investigations necessitate subjecting specimens to accelerated corrosion and measuring the degree of corrosion. “However, measuring the effect of corrosion inhibitors in actual structures using a destructive method is extremely difficult.”
Sensors that can measure changes in electrical resistance caused by iron corrosion and thus indicate the degree of corrosion of material have recently been developed. These sensors offer a non-destructive method of assessing the degree of corrosion in concrete materials. As a result, the effect of Lithium Nitrite as a corrosion inhibitor has been studied non-destructively.
Health Hazards
When inhaled, lithium nitride can have an effect on you, and it can also be absorbed through the skin. Contact can cause severe irritation and burns to the skin and eyes. Breathing Lithium Nitride can cause nose and throat irritation.