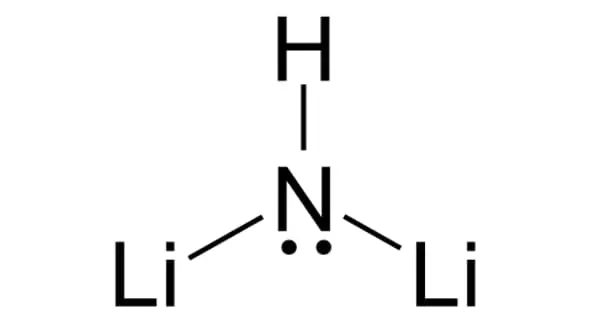
Lithium imide is a chemical compound with the formula Li2NH. It is a white crystalline powder with an ammonia odor. A reaction between lithium amide and lithium hydride can produce this white solid.
LiNH + LiH → Li2NH + H2
The product is light-sensitive and can undergo disproportionation to form lithium nitride, which is characteristically red.
2 Li2NH → LiNH2 + Li3N
Lithium imide is thought to have a simple face-centered cubic structure with an Fm3m space group; with N-H bond distances of 0.82(6) Å and an H–N–H bond angle of 109.5°, giving it a similar structure to lithium amide.
Because of the very localized negative charge on the nitrogen, which carries two formal charges, lithium imide is strongly basic and deprotonates even some extremely weak acids such as methane and ammonia. It finds application in organic and organometallic chemistry. It has been studied as a hydrogen storage material.
Preparation
In the first step, lithium metal is reacted with ammonia to form lithium bronze, and in the second step, the lithium bronze is reacted with a 1,3-diene or an arylolefin in the presence of a solvent to form lithium amide.
The invention relates to a method for preparing lithium amide.
Lithium amide is a powerful base. It is frequently used as a reagent in synthetic organic chemistry, such as condensation and alkylation reactions. The lithium metal is typically dissolved in liquid ammonia (T < −33° C.) and then reacted under the catalysis of a transition metal compound to form lithium amide. The low temperature and hydrogen formation are disadvantages of this operation. At temperatures above 400° C., the reaction of lithium metal and gaseous ammonia is also known. The disadvantage is the high temperature and the formation of hydrogen.
The goal of the invention is to overcome the disadvantages of the prior art, specifically to provide a method for preparing lithium amide that emits no hydrogen and operates as close to ambient temperature as possible.
The heat of reaction in the second reaction step can be controlled by changing the rate dosage of the 1,3 diene or arylolefin, for example. The resulting lithium amide is insoluble and heavier than the reaction solution. The solvent dissolves the hydrogenated 1,3 diene or arylolefin; the ammonia is discharged in gaseous form and can be reclaimed. The powdery lithium amide is separated from the solvent and dried if necessary.
The advantage of the method described in the invention is that the reaction can be carried out at room temperature (without subjecting the product to thermal loading), that the product is purer (impurities can be separated with the solvent), that no hydrogen is formed, and that the hydrogen-free ammonia can be recovered.