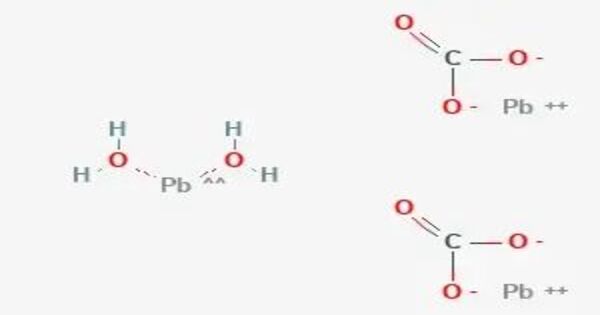
Lead(II) carbonate is the chemical compound with the chemical formula PbCO3. It is a white solid with several practical uses, despite its toxicity. It occurs naturally as the mineral cerussite. It is also known as plumbous carbonate.
Lead(II) carbonate is a white solid at room temperature. It is practically insoluble in water, meaning it does not dissolve easily. Historically, it was used as a white pigment in paints, though this usage has declined due to concerns over lead toxicity. It has also been used in some traditional medicines, but again, this is problematic due to lead’s toxicity.
Properties
- Chemical formula: PbCO3
- Molar mass: 267.21 g/mol
- Appearance: White powder
- Density: 6.582 g/cm3
- Melting point: 315 °C (599 °F; 588 K) (decomposes)
- Solubility in water: 0.00011 g/(100 mL) (20 °C)
- Solubility product (Ksp): 1.46·10−13
- Solubility: insoluble in alcohol, ammonia; soluble in acid, alkali
Production and use
Lead carbonate is manufactured by passing carbon dioxide into a cold dilute solution of lead(II) acetate, or by shaking a suspension of a lead salt more soluble than the carbonate with ammonium carbonate at a low temperature to avoid formation of basic lead carbonate.
Pb(CH3COO)2 + [NH4]2CO3 → PbCO3 + 2 [NH4](CH3COO)
Preparation
Lead(II) carbonate can be prepared by reacting lead(II) nitrate with sodium carbonate or sodium bicarbonate in solution.
Lead carbonate is used as a catalyst to polymerize formaldehyde to poly(oxymethylene). It improves the bonding of chloroprene to wire.
Toxicity
Lead is highly toxic to humans and can cause serious health problems, especially if ingested or inhaled over time. This toxicity has led to strict regulations regarding its use and disposal.
Due to its toxicity, the use of lead(II) carbonate has been heavily restricted and regulated in many countries. Alternatives have been developed for its former applications in paints and other areas where its toxicity posed a risk to human health and the environment.