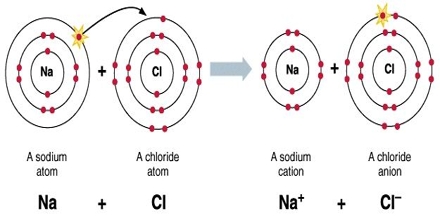
It is important to recognize that clean ionic bonding – by which one atom “takes” a great electron from yet another – cannot exist: All ionic compounds possess some degree of covalent bonding, or electron discussing. Ionic bonding is a variety of chemical bond that needs the electrostatic interest between oppositely charged ions. Ionic bonding can result from a redox reaction when atoms of an element (usually metal), give some of their electrons to achieve a stable electron configuration.
Ionic Bonding