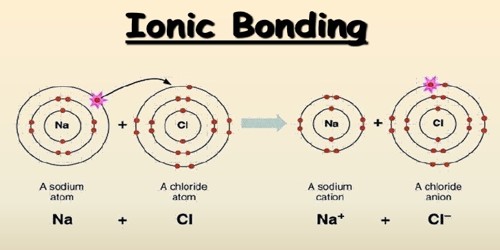
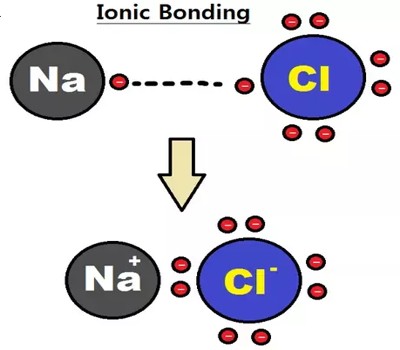
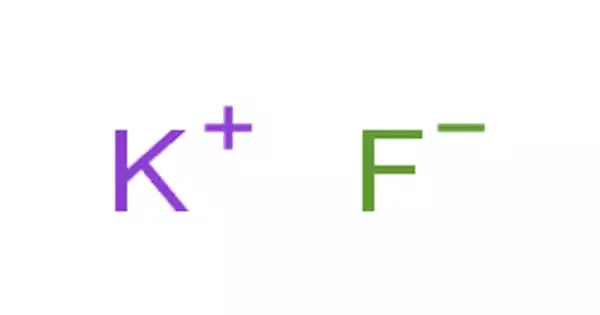An ionic bond is the electrostatic forces of attraction between a non-metal and a metal ion in a giant ionic crystal lattice. It is a type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. This occurs when charged atoms (ions) attract. Ionic bonds require at least one electron donor and one electron acceptor. This happens after a metal atom loses one or more of its electrons to the non-metal atom. The greater the difference in charge between the metal and non-metal ion, the stronger the ionic bond. It is a type of chemical bond that generates two oppositely charged ions. A maximum of three electrons can be transferred in the process. Ionic bonding is observed because metals have few electrons in their outer-most orbitals. They are formed between cations and anions.
An ionic bond is based on attractive electrostatic forces between two ions of opposite charge. A metal atom becomes a positive action because it loses electron(s). A non-metal atom becomes a negative anion as it gains an electron(s). By losing those electrons, these metals can achieve a noble gas configuration and satisfy the octet rule. This occurs, for example, when sodium and chlorine join to form common table salt, NaCl. First, sodium atoms (Na) oxidize and lose an electron to form positively charged sodium ions (Na+). The charges on the anion and cation correspond to the number of electrons donated or received. Chlorine atoms gain the electrons from the sodium atoms to form negatively charged chloride ions (Cl-). Both ions are now oppositely charged and they are held by strong electrostatic forces of attraction. In ionic bonds, the net charge of the compound must be zero.
Features of ionic bonds
- The three-dimensional ionic structure called a giant ionic crystal lattice structure.
- Ionic compounds are soluble in water as the ions form favorable interactions with water molecules which release sufficient energy to break away from the lattice.
- In a solid-state, they do not conduct electricity. However, in a liquid state or when dissolved in water, they will conduct electricity well because the ions are free to move and can carry a charge.
- The contrast to the characteristics of a covalent bond.
- Sometimes if they do not have a spare valence electron to create a complete shell one will act as two and spin in a figure of eight around both atoms.
- Ionic bonds are generally a lot weaker than covalent bonds.
- Ionic compounds have high melting/boiling point due to the strong electrostatic forces of attraction, which require a large amount of heat energy to overcome.