Researchers made a significant finding in the field of cellular microscopy. The team has successfully developed Two-Color Infrared Photothermal Microscopy (2C-IPM), a unique method for studying neutral lipids within living cell lipid droplets. This novel microscope can be combined with isotope tagging to enable thorough monitoring of neutral lipid production within individual lipid droplets.
South Korean researchers at the IBS Center for Molecular Spectroscopy and Dynamics (IBS CMSD), lead by Director CHO Minhaeng, produced a ground-breaking breakthrough in the field of cellular microscopy. The team has successfully developed Two-Color Infrared Photothermal Microscopy (2C-IPM), a unique method for studying neutral lipids within living cell lipid droplets. This novel microscope can be combined with isotope tagging to enable thorough monitoring of neutral lipid production within individual lipid droplets.
Lipid droplets (LDs) are structures made up of pockets of neutral lipids (triglycerides) enclosed in a monolayer of phospholipids. They have long been regarded as uninteresting organelles whose sole function is to store excess energy in the form of neutral lipids. However, new research suggests that these droplets have an active role in a variety of cellular metabolic functions. It was shown that they play an active role in controlling lipid toxicity and cell communication, as well as their association with common disorders such as obesity and nonalcoholic fatty liver disease. Understanding the activities of LDs is therefore critical for diagnosing and treating these diseases.
By successfully observing the process of neutral lipid synthesis in living cells, we have laid a new foundation for studying the molecular functions of lipid droplets within cells. This approach is likely to be widely applied in the study of numerous cellular metabolic phenomena.
CHO Minhaeng
Researchers have historically stained cells with lipophilic dyes and used fluorescence microscopy to analyze LDs within them. However, this approach has several important disadvantages. The first step is dye photobleaching, which limits the LDs’ observation period to relatively limited time windows. Another constraint is the fluorescent dyes themselves. The currently employed fluorescent dyes only target the hydrophobic environment of LDs, using undefined binding methods. As a result, they are unable to appropriately assess the content and quantity of neutral lipids.
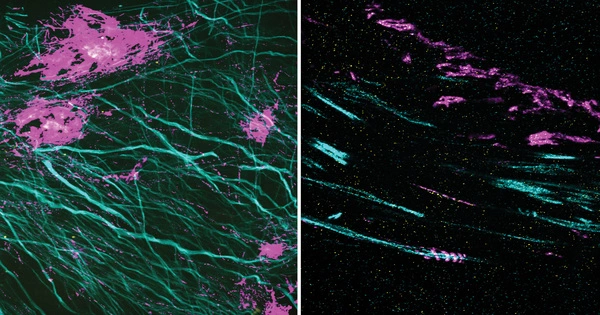
Unlike previous methods that relied on fluorescent microscopy, the new 2C-IPM technology uses an infrared (IR) spectroscopic method and does not require the use of fluorescent dyes. The new method monitors neutral lipids within LDs directly by detecting changes in IR absorbance. Importantly, this advantage allows researchers to observe the synthesis of neutral lipids within individual LDs in living cells over a long period.
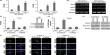
Employing the newly developed method, the research team studied the synthesis of neutral lipids in cells when they were exposed to excess fatty acids. The researchers were able to distinguish freshly synthesized neutral lipids from pre-existing neutral lipids within cells by subjecting deuterium-labeled fatty acids, which have distinct spectroscopic properties from non-deuterated forms. The analysis results verified excess fatty acids caused lipid toxicity, and cells respond by increasing the synthesis of neutral lipids.
The primary author, researcher PARK Chanjong, stated, “This study serves as a fundamental example of the possibility of long-term research on lipid droplets and internal neutral lipids in living cells. The analytical method used in this study can be used to diagnose and treat disorders closely related to lipid metabolism, such as nonalcoholic fatty liver disease.”