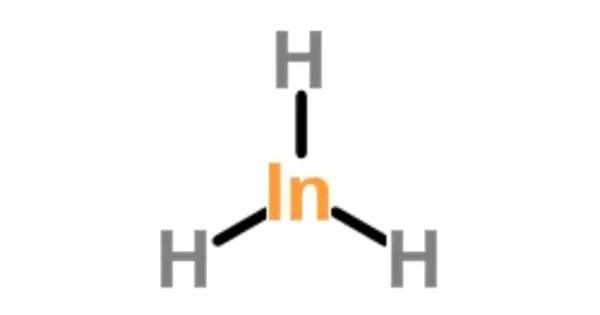
Indium trihydride is an inorganic compound with the chemical formula (InH3). It is an inorganic compound that has been studied primarily in theoretical chemistry and materials science. While it has not found widespread practical applications, its properties and occurrences are of interest in specific scientific contexts.
It has been observed in matrix isolation and laser ablation experiments. Gas phase stability has been predicted. The infrared spectrum was obtained in the gas phase by laser ablation of indium in presence of hydrogen gas InH3 is of no practical importance.
Properties
As a solid, InH₃ is likely to be a colorless or white compound, but in practice, the compound is not isolated easily due to its instability. It is highly unstable and tends to decompose into its constituent elements or form other more stable indium-hydrogen compounds (like InH or In₂H₃) when exposed to air or light. It is likely to be soluble in organic solvents and reactive in water, but due to its instability, it’s not commonly encountered in bulk form.
- Chemical formula: InH3
- Molar mass: 117.842 g/mol
- Melting point: −90 °C (−130 °F; 183 K) (decomposes)
Chemical properties
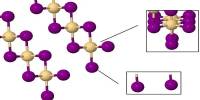
Solid InH3 is a three-dimensional network polymeric structure, where In atoms are connected by In-H-In bridging bonds, is suggested to account for the growth of broad infrared bands when samples of InH3 and InD3 produced on a solid hydrogen matrix are warmed. Such a structure is known for solid AlH3. When heated above −90 °C, indium trihydride decomposes to produce indium–hydrogen alloy and elemental hydrogen.
Synthesis
- Indium Trihydride is not commonly synthesized on a large scale due to its instability. However, it can theoretically be produced by reacting indium metal with hydrogen gas under controlled conditions (high pressure, low temperature).
- A method for the preparation of indium hydrides might involve the direct reaction of indium with hydrogen in a specialized environment to prevent decomposition.
Occurrences in Nature
- Natural Occurrence: Indium Trihydride is not a naturally occurring compound. Indium itself is a rare element found mainly in ores like sphalerite, often as a byproduct of zinc extraction. Indium hydrides, including InH₃, are laboratory-created compounds.
- Occurrence of Indium: Indium is found in trace amounts in Earth’s crust and is often recovered from zinc ores or from the recycling of electronic waste. It is primarily used in electronics, particularly in the production of thin-film solar panels and liquid crystal displays (LCDs).
Uses and Applications
- Limited Applications: Due to its instability, Indium Trihydride does not have significant applications in industry or technology. Most uses of indium are in more stable compounds, such as indium tin oxide (ITO), which is used in electronic and display technologies.
- Research: Indium hydrides are of interest in research fields, especially in studying the chemistry of hydrides, metal-hydrogen interactions, and the behavior of low-valent indium species.
Safety and Handling
Because Indium Trihydride is highly reactive and unstable, it would be hazardous to handle without proper precautions. It can decompose violently in the presence of air or moisture, producing hydrogen gas, which is flammable and could lead to explosive conditions. Standard safety measures when handling hydrides, such as working in a fume hood and using proper protective equipment, would be necessary.