Hypochlorite is an ion. It is a salt of hypochlorous acid. It is a chlorine oxoanion, a chlorine oxide, and a monovalent inorganic anion. It has a chlorine odor and becomes more unstable at higher temperatures. It is very unstable, but hypochlorous acid is stable and is highly microbicidal, active against bacteria, viruses, and fungi. It is a chemical compound containing the molecular ion of chlorine and oxygen and a counter-ion such as sodium or calcium.
Hypochlorites react with acids to produce chlorine. Their chemical formula is ClO-. It is a conjugate base of hypochlorous acid. It is a strong oxidizing agent. It contains chlorine in its +1 oxidation state. They are the salts of hypochlorous acid. It is an active ingredient of household bleach and is also produced by myeloperoxidase from activated macrophages during the infection process. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of bleaching powder, swimming pool “chlorine”). Even though hypochlorites are alkaline, they are the strongest oxidizing agents of all compounds containing chlorine oxyanions.
It can be found in household bleach, sodium hypochlorite. Their primary applications are bleaching, disinfection, and water treatment agents, but they are also used in chemistry for chlorination and oxidation reactions. It is unstable, meaning that it breaks down easily. It is often used as a chemical reagent for chlorination and oxidation reactions.
Common hypochlorites include:
- Sodium-hypochlorite
- Calcium-hypochlorite
- Barium-hypochlorite
- Lithium-hypochlorite
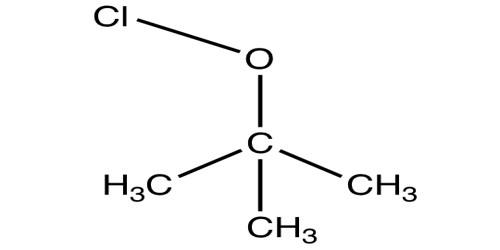
- Methyl-hypochlorite.
Applications include:
- Bleaching
- Disinfection and water treatment
- Chlorination and oxidation
- Sanitization.