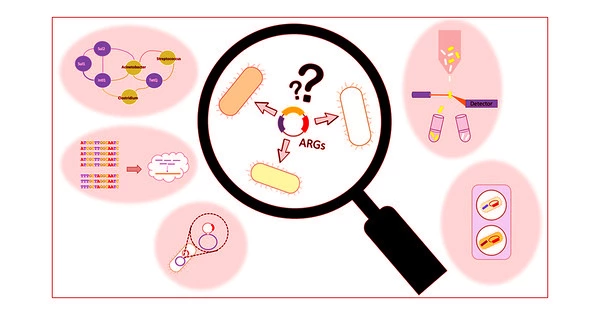
Bioscientists discover how to activate ‘silent’ gene clusters in bacteria, which could be a goldmine for new antibiotic candidates. Silents could be gold in the search for antibiotics to slow the ongoing resistance crisis in disease treatment.
Rice University bioscientists created new on/off switches to control “silent” genes in a bacterial strain. Their strategy has the potential to accelerate the ongoing search for new antibiotics. The researchers modified CRISPR tools to control the expression of genes in Streptomyces bacteria that are normally expressed only when necessary in nature. Until now, access to those genes has been difficult for synthetic biologists.
“As labs started to sequence the genomes of these organisms that were known to produce one or a few antibiotics, we realized that the pathways responsible for the production of antibiotic and other molecules of interest are much more abundant than previously thought,” said James Chappell, an assistant professor of biosciences whose lab studies bacteria and ways to engineer them.
“Each Streptomyces strain is now predicted to be able to produce up to 40 different molecules of interest, including antibiotics, on average,” he said.
Streptomyces is a bacterial genus that contains up to 500 species, and each species can have between 20 and 40 of these clusters of genes capable of producing antibiotics or other molecules of interest. So, once we figure out how to scale up our technology, it has the potential to be extremely powerful.
James Chappell
The research led by Chappell and graduate student Andrea Ameruoso may enable labs to rapidly develop libraries of potential antibiotics to test on pathogens. They also stated that, while CRISPR-Cas9 has been used to create a platform for activating genes in organisms such as E. coli, this is the first time it has been applied to Streptomyces.
Their findings were published in Nucleic Acids Research.
“Bacteria like Streptomyces have evolved to produce antibiotics only when necessary, in natural environments like soil,” Chappell explained. “Because we grow them in the lab in an artificial environment that is very different from how they grow naturally, sets of genes are silenced.”
“They’re a kind of genetic dark matter,” he said. “We can’t isolate the chemicals they express to perform a functional screen.”
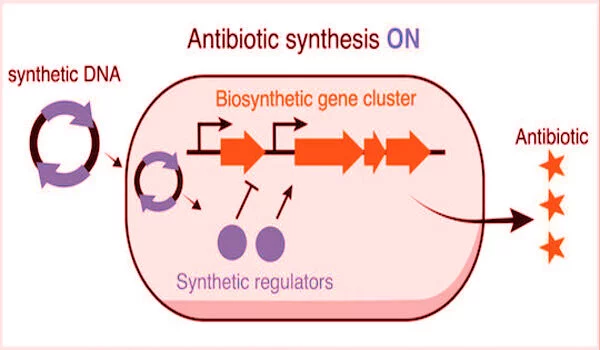
The new strategy of the lab eliminates the time-consuming task of exposing their proof-of-concept bacteria, S. venezuelae, the source of the common antibiotic chloramphenicol, to potential gene expression triggers. “Andrea’s technology introduces synthetic regulators into cells to artificially stimulate or suppress the expression of these pathways,” Chappell explained.
“Now we only need one protein and one small piece of RNA to go wherever we want to directly repress or activate a given target,” Ameruoso explained. According to him, the emergence of CRISPR technology, which uses bacterial immune system mechanisms to locate specific genes along a strand of DNA, facilitated access to previously hidden gene clusters.
“Streptomyces is a bacterial genus that contains up to 500 species, and each species can have between 20 and 40 of these clusters of genes capable of producing antibiotics or other molecules of interest,” Ameruoso explained. “So, once we figure out how to scale up our technology, it has the potential to be extremely powerful.”
According to Chappell, it is simple to design CRISPR to bind to different DNA sequences. “We use that to control gene expression,” he says. “It should be theoretically possible to do this in a variety of different species via a variety of different pathways. As a result, this paper establishes the groundwork for a novel approach.”
Ameruoso stated that he is working on a fluorescent technique to observe cluster activation in real time. “The main challenge is that observing the depths of a cluster’s activation is dependent on the purification of the molecule from the extracts we generate,” he explained. “This is a labor-intensive, low-throughput process. We want to create a reporter that will detect a fluorescent signal when a pathway is activated.”
The process, according to the researchers, could be used to create molecules for antifungal and anticancer agents, as well as for agricultural purposes. “We concentrate on antibiotics because we’ve observed that they kill microbes at some point in history,” Chappell explained. “But that’s not necessarily what they evolved for, because they’re also frequently used as communication signals between cells. So there are many potential uses.”
According to him, the study demonstrates a significant new approach to the activation of silent pathways. “The next generation of work is going to be big,” he said. “We demonstrated that it works on a single silent pathway. Let’s try it on the 40 pathways in this single species, and then on thousands of microbes. The power of CRISPR-Cas9 is that it is extremely scalable for that,” Chappell explained.