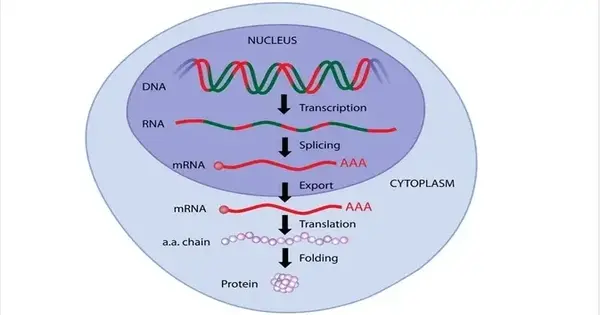
To work effectively, the genetic material is highly structured into loop structures that frequently bring together previously dispersed portions of the genome that are crucial to gene regulation. Scientists are now investigating how these loops can help suppress or stop gene activity, with potentially far-reaching implications for human health.
Each cell’s nucleus contains the DNA that contains the blueprint for human life. In human cells, around six and a half feet of genetic material must be condensed to fit within the nucleus. DNA condensation is not a random process. To work effectively, the genetic material is highly structured into loop structures that frequently bring together previously dispersed portions of the genome that are crucial to gene regulation.
In a new paper published in Nature Communications, USC Stem Cell scientists from the laboratory of Oliver Bell address how these loops can help repress or silence gene activity, with potentially far-reaching effects on human health.
Our discovery is noteworthy because it demonstrates a dependence of PRC 1 and PRC 2 activity on the precise regulation of 3D genome architecture by cohesin, implying that ‘cohesinopathies’ may be linked to abnormal embryonic gene silencing.
Daniel Bsteh
“A carefully orchestrated regulatory machinery is required to ensure every cell in the body is expressing its correct gene set to exert its dedicated function,” said the study’s first author Daniel Bsteh, who began the research at the Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA), and completed it at the Keck School of Medicine of USC during his PhD. He is currently the Liquid Biopsy Core Manager at the USC Norris Comprehensive Cancer Center.
Bsteh and his colleagues focused on developmental genes that are inhibited by molecules known as Polycomb Repressive Complexes 1 and 2 (PRC1 and PRC2). PRC1 and PRC2 are regulators that prevent developmental genes from being activated at the incorrect time or in the wrong cell, which has been proven to cause alterations in cellular identity, resulting in developmental abnormalities or cancer cell transformation.
When PRC1- and PRC2-repressed genes are combined, the genome generates loops. Loops are recognized to have a role in gene activation, but it has been more difficult to investigate how loops may assist repress genes. This is due to loop interaction with histone changes, a new form of gene repression mechanism.
Through a genetic screen conducted in mouse embryonic stem cells, the scientists identified a protein, PDS5A, that modifies loops without affecting histone modifications. This enabled Bsteh and colleagues to specifically study the effects of loops and 3D genome organization on gene silencing.
The loss of PDS5A disrupted the loops — and therefore the long-range interactions between repressed developmental genes. Further, looping genes together maintains the silent state. When PRC1- and PRC2-repressed genes are physically separated, eliminating the loops, normally silent genes become activated in aberrant ways.
“PDS5A is a subunit of a larger protein complex called cohesin, which is the master regulator of 3D genome organization,” explained Bell, an assistant professor of biochemistry and molecular medicine, stem cell biology and regenerative medicine, and a member of the USC Norris Comprehensive Cancer Center.
“Cohesin mutations are known to cause a variety of human diseases, including developmental disorders and cancer. Our discovery is noteworthy because it demonstrates a dependence of PRC 1 and PRC 2 activity on the precise regulation of 3D genome architecture by cohesin, implying that ‘cohesinopathies’ may be linked to abnormal embryonic gene silencing.”