Introduction
It has been estimated that 2.2% of the world population that is 130 million individuals are infected with hepatitis C. It represents a viral pandemic, one that is five times as widespread as infection with the human immunodeficiency virus type-1 (HIV-1). Hepatitis C is now one of the most common liver disease, is having overtaken alcohol included liver disease in the past few years around the world2.
Hepatitis C infection may cause a benign, a symptomatic disorder with an indolent course but it may also cause progressive liver disease, cirrhosis and primary liver cancer. The illness has a complex, natural course and the ultimate, long term prognosis for patients with chronic hepatitis is difficult to predict1.
Viral hepatitis occurs throughout the world. Of the seven viral hepatitis viruses so far recognized, hepatitis C virus remains the largest global challenge3. Commonly occurring hepatotropic viruses are Hepatitis A virus (HAV), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Hepatitis D virus (HDV), Hepatitis E virus (HEV) and Hepatitis G virus (HGV). The list of potential hepatotropic viruses continues to grow, with the recent discovery of GB virus-C (HGV), the TT virus and the SEN virus. Both GB virus C and TT virus has global distribution. The SEN virus is thought to be a novel viral agent that may be linked to cryptogenic chronic hepatitis, but data are awaited4. The HAV, HCV, HDV, HEV, and HGV are RNA viruses and HBV is a DNA virus5. The HAV and HEV are nonenveloped and enterically transmitted and cause acute and self limited disease only. Whereas the main route of infection of HBV, HCV, HDV and HGV is parenteral and can cause persistent infection and chronic hepatitis and in cases of HBV and HCV, hepatocellular carcinoma6. Faecal oral transmission does not occur with these viruses because their lipid envelopes preclude the passage of viable virus from the liver through the biliary system to intestinal tract, as occurs with HAV and HEV.
Hepatitis C virus (HCV) was identified and partially characterized as recently as 19897. Since then, the entire viral genome has been sequenced, its genotypes have been determined, the structural and non structural proteins have been defined and the virus has been classified as a member of the flavivirus family. HCV is the most common cause of post transfusion and community acquired non-A, non-B hepatitis and cryptogenic cirrhosis world wide5.
The institution of blood screening measures in developed countries has decreased the risk of transfusion-associated hepatitis to a negligible level8. But new cases continue to occur mainly as a result of injection-drug use and, to a lesser degree, through other means of percutaneous or mucous membrane exposure. Infection with the virus has become the main indication for liver transplantation 8. The infection is usually chronic and commonly silent for many years. Hepatitis C virus infection results in anicteric hepatitis in 75% cases and fulminant hepatic failure are rare in acute hepatitis9. The virus has a striking serologic association with HCC and is a leading cause of end stage liver disease requiring liver transplantation. With the availability of more reliable assays HCV infection is emerging as an extremely common and insidiously progressive disease that may result in chronic active hepatitis, cirrhosis and HCC. The prevalence of antibodies to HCV in the US general population is 1.4%’°. Whereas 2% to 5% of adult patients with acute hepatitis B infection developed chronic hepatitis B and an estimated 50% to 75% of patients with acute hepatitis C develop chronic infection10,55. 20 percent of persons with chronic hepatitis C will eventually have cirrhosis, and this can occur 20 years or less after infection, especially in those with alcohol abuse or co-infection with HIV virus type-1 or hepatitis B virus. Once cirrhosis is established, the risk of hepatocellular carcinoma is 1 to 4 percent per year 10. Moreover, chronic HCV infection rarely, if ever spontaneously resolves. Although research advances have been impeded by the inability to grow HCV easily in culture, there have been new insights into pathogenesis of the infection and improvements in treatment options.
Historical Background
Following the development of serological tests for the diagnosis of hepatitis A and B, it became evident in the mid-1970s that there were many cases of infectious hepatitis unrelated to either agent10,11. Since the time of Hippocrates, viral hepatitis has been a part of recorded history. In 1885, events in Bremen, Germany, suggested a variant of acute epidemic hepatitis not previously described12. Nearly 200 shipyard workers became jaundiced several months after vaccination against smallpox with human lymph. Lurman’s description of these cases and the absence of jaundice in workers who were not vaccinated with the same material leave little doubt that their illness was long-incubation hepatitis that was parenterally transmitted by contamination of the lymph inoculum with blood. The absence of secondary cases among co-workers, spouses, and children makes it likely that this was the first good description of parenterally transmitted non-A, non-B hepatitis (NANBH). During the early 20th century, epidemics of blood-borne hepatitis were described in patients attending venereal disease, diabetes, and arthritis clinics where they received injection treatments; in children who received inoculations of human convalescent serum for protection against measles and mumps, in recipients of blood products; and in military personnel who were inoculated with the yellow fever vaccine.
Further progress in the understanding of viral hepatitis was not made until the large number of hepatitis cases occurring during World War II drew attention to the morbidity of the disease and justified human transmission experiments12. Human investigations performed at Willow brook State School for the mentally retarded took advantage of the high risk of spontaneous hepatitis in this institutionalized population to confirm and extend previous observations about the natural history, epidemiology, and prevention of acute viral hepatitis.
HBV was isolated by immunologic techniques visualized by electron microscopy, and serologically characterized by 3 antigen-antibody systems. The discovery of the Australia antigen by Blumberg and colleagues in 1965 and its subsequent association with hepatitis B virus (HBV; serum hepatitis) began a period of accelerated hepatitis research. Transmission studies in subhuman primates and people permitted detailed descriptions of the clinical and histologic manifestation of the infection and allowed the distinctions between epidemic (infectious) and blood-borne (serum) hepatitis” to be more carefully described. Application of the serologic tests for HBV indicated that a significant proportion of transfusion-associated hepatitis could not be accounted for by this virus. The long incubation period for post-transfusion hepatitis did not support a role of the infectious hepatitis agent. Finally, the discovery by Feinstone et al in 1973 of hepatitis A virus, the agent responsible for infectious hepatitis, failed to explain the many indefinable cases of post-transfusion hepatitis. Elimination of hepatitis B surface antigen-positive blood donors did not significantly alter the total number of cases of post-transfusion hepatitis.
The name “non-A, non-B (NANB) hepatitis” was coined for a diagnosis based upon exclusion criteria, in the absence of specific assays for the etiological agent(s)10. In the late 1970s and early 1980s it became clear that NANB infection was responsible for 15 to 50 per cent of all cases of acute viral hepatitis in adults and that major risk factor for community acquired NANB hepatitis were blood transfusion, use of parenteral drugs, and occupational (e.g. health personnel) contact with infected patients10. It is also became obvious that about 40 per cent of all patients in the community with acute NANB hepatitis had no overt parenteral exposure to contaminated materials or other risk factors which could explain acquisition of the disease10. Thus, the concept of NANBH developed in the early 1970s after the identification of hepatitis A and B made it obvious that many cases of hepatitis, particularly post-transfusion hepatitis, could not be accounted for by either of these viruses. An important break through in identifying the cause of NANBH was the demonstration of transmission of NANBH to chimpanzees by injecting them with human serum or plasma from patients with hepatitis and donors who had previously been implicated in the transmission of the disease.
Break Through in the History of Hepatotropic Viruses4,6,10
| 1965 | Discovery of the hepatitis B surface antigen (Blumberg). |
| 1973 | Identification of the hepatitis A virus (Feinstone). |
| 1975 | Description of multiple episodes of hepatitis in same patients (Mosley). |
| 1975 | Proposal of existence of “non-A, non-B hepatitis” (Feinstone). |
| 1977 | Documentation of transmissible agent (Hoofnagle). |
| 1980 | Successful chemical inactivation of NANB agents. |
| 1986 | First evidence of therapeutic effect of alpha interferon (Hoofnagle). |
| 1989 | Identification and cloning of the hepatitis C virus (Choo). |
| 1990 | Introduction of anti-HCV testing in blood donors and clinics. |
| 1990 | Food and Drug Administration approval of recombinant interferon alpha-2b for treatment of chronic hepatitis C. |
| 1990 | Discovery of hepatitis E virus. |
| 1996 | Discovery of hepatitis G virus (GBV-C). |
| 1997 | First reporting of TT virus (TTV) – a novel unenveloped ss DNA virus. |
Several investigators identified ultrastructural changes in the livers of infected patients and chimpanzees. The changes were not viral particles but ultrastructural cellular changes induced by infection. Cross-challenge experiments and the presence of mutually exclusive nuclear or cytoplasmic changes after inoculation with 2 different NANBH strains (strains H and F) suggested that more-than one NANBH agent existed. However logical these original conclusions seemed at the time, they were disproved by experiments more than a decade later showing that strains H and F were variants of the same virus. Other investigators reportedly identified the virus by electron microscopy, although none of the studies could be independently confirmed.
Feinstone et al first reported about the existence of a NANB virus in 1975 and after 14 years of this proposal, Choo discovered the hepatitis C virus in 198910. Data were collected indicating that most patients with chronic NANB (or cryptogenic) liver disease were positive for anti HCV including those with cirrhosis and hepatocellular carcinoma, leading to the conclusion that HCV was the causative agent in the vast majority of non alcoholic, non-autoimmune HBsAg negative cases of chronic liver disease. This break through was a milestone in the history of medical science leading to the recognition of NANBH as hepatitis C.
a. Animal infectivity model
Some of the physical characteristics of HCV are known even though HCV itself has not been visualised. This has been achieved partly by means of an animal infectivity model. In 1978, the chimpanzee was the only animal found to be susceptible to NANB infection, with associated elevation of serum alanine aminotransferase (ALT) and some histological evidence of hepatocellular inflammation, thus proving that this form of hepatitis was caused by a transmissible agent16.
The size of the putative hepatitis C virus has been determined indirectly by ultrafiltration of infectious plasma through membranes with precisely graded pore sizes. The inoculation of chimpanzees or amplification of viral RNA using PCR techniques were used to confirm the presence of virus in the ultrafiltrate. These studies indicated that the virus particle has a diameter of less than 80 nm. It is possible that uncoated core particles were being measured in the latter observation, where the virus was estimated to have a diameter of between 30-38 nm. The buoyant density of HCV, previously reported as 1.09-1.11 g/ml, has recently been confirmed as 1.115 g/ml. Chloroform sensitivity studies revealed that the virus had an outer lipid envelope16.
b. Cloning
For several years scientists failed to identify the causative agents of NANB hepatitis using conventional immunological techniques. Eventually, in 1989, developments in recombinant molecular technology
allowed a research group at Chiron Corporation, in association with the CDC, to announce the discovery of a viral antigen expressed from a bacteriophage lambda GT-11 library, which appeared to be related to at least one form of NANB hepatitis; the responsible agent was designated hepatitis C virus16.
Relationship between HCV and the flaviviridae family
The genome of the hepatitis C virus (HCV) comprises a positive-stranded RNA molecule of about 9,500 nucleotides containing a long translational open reading frame (ORF) that encodes a large polypeptide of approximately 3,000 aminoacids beginning with the first in-frame methionine codon6,10.
The 5′ terminus of the RNA genome has substantial primary sequence identity with the corresponding region of the pestivirus genomes6,10, and a region of the encoded polypeptide exhibits significant sequence identity with nucleoside triphosphate binding helicases encoded by the pestiviruses and to a lesser extent, the flaviviruses6,10. Protease and replicase sequence motifs conserved among the pestiviruses and flaviviruses are also present within the HCV encoded polyprotein, which, along with the more extensively conserved helicasc sequence, are all similarly colinear among the three types of viral polyproteins. Although these are the only regions of HCV exhibiting significant primary sequence identity with the pestiviruses and flaviviruses, the hydropathicity of the HCV-encoded polypeptide is remarkably similar to that of the flaviviruses and, to a lesser extent, the pestiviruses, thus indicating similarities in their basic structures and
functions6. Three new flavilike viruses, GBV-A, GBV-B, and GBV-C (also known as HGV), have recently been identified4‘6‘13. Nucleotide and protein sequence analyses show that these three viruses are more closely related to each other and HCV than to the other members of the Flaviviridae family. HCV and GBV-A, -B, and C appear to form a discrete cluster of related viruses within the larger genus of flavivirus. It has been established that HCV is the major etiological agent associated with post-transfusion non-A, non-B hepatitis (NANBH), as well as being a major cause of sporadic NANBH.
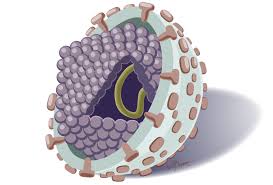
Structure Of The Virus The HCV virion
Analysis of the structure of HCV particles has been hampered by the low titer of virus in infectious sera and the difficulties of replicating the virus in culture systems. Particles have been observed by electron microscopy in human plasma, chimpanzee liver, and experimentally infected or transfected cell lines. The possibility that HCV particles are present in the circulation as immune complexes or in association with serum lipoproteins has also been suggested13.
The hepatitis C virus is 30-60 nm in size and is an eveloped, linear single stranded, positive sense, 9500 nucleotide RNA virus, the genome of which is similar in organization to that of flaviviruses and pestiviruses. HCV constitutes its own genus in the family flaviviridae. HCV replication has been demonstrated in hepatocytes and in extrahepatic sites including peripheral blood mononuclear cells6,10,13.
Features of hepatitis viruses 8,10
Type | Virus particle | Morphology | Genome | Classification | Antigen | Antibodies | Remarks |
| HAV | 27nm | Icoshedral noneveloped | 7.5.kb RNA linear. ss+ | Hepatovirus | HAV | anti-HAV | Early faecal shedding Diagnosis: 1gM anti-HAV. Previous infection: IgG anti HAV. |
| HBV | 42nm | Double-shelled virion (surface and core spherical) | 3-2 kb DNA, circular, ss/ds | Hepadnavirus | HBsAg HBcAg HBcAg | anti-HBs anti-HBc anti-HBe | Bloodborne virus, earner states Acute diagnosis: IIBsAg. igM aim IIBe Chronic diagnosis IgM anii-IIBc. HBsAg Markers of replication: IlkAg IIIW DNA Liver, lymphocytes, other organs |
| 27nm | Nucleocapsid | HBcAg HBeAg | anti-HBc anti-HBe | Nuclcocapsid contains DNA and DNA polymerase; present in hcpatocyte nucleus; HBcAg does not circulate; IIBeAg (soluhel, nonparticulatc) and HIW DNA circulatc-correlatc with in 1’ecti vit\ and complete vinous. | |||
| 22nm | Spherical and fimalentous; represents excess virus coat material | HBsAg | anti-HBs | HBsAg detectable in >95% of patients with acute hepatiti B; found in serum, body fluids. hcpatocyte cytoplasm; anli-IIBs appears following infection-protective antibody | |||
| HCV | Approx 30-60 mm | Enveloped | 9.4-kb RNA Linear.ss+ | Flavivirus like | HCV C100-3 C33c C22-3 NS5 | anti-HCV | Bloodborne agent, formerly labeled non-A. non-B hepatitis Acute diagnosis anti-HCV (C33c. C22-3. NS5) and IICV RNA Chrome diagnosis anti-HCV (C100-3. C33c. C22-3e. NS5) and IK V KNA. cytoplasmic local ion in heaptocytes |
| HDV | 35-37nm | Enveloped hybrid particle with HBsAg coat and HDV core | 1-7-kb RNA circular, ss | Resembles viroids and plant satellite viruses | HBsAg HDV antigen | anti-HBs Anti-HDV | Defective KNA virus, requires helper function of HBV (hepadnaviruses); HDV antigen present in hepatocyte nucleus. Diagnosis: anti-HDV. HDV RNA. HIW/HDV comfection-IgM andti-l IBc and anti-HDV; HDV super infection IgCi anti-HBc and anti-HDV. |
| HEV | 33-34nm | Noneveloped icosa hedral | 7-6-kb RNA Lnear.ss.+ | Alphavirus like | HEV antigen | anti-HEV | Agent of ciUerically transmitted hepatitis, rare in USA; occurs in Asia. Mediterranean countries. Central America Diagnosis IgM/lgCi anti-IIIA (assays being developed), \inis in stool. bile. hepatoeuc cytoplasm |
ss, single stranded; ss/ds, partially single-stranded, partially double stranded;- minus stranded;+plus stranded.
HCV proteins6
Protein | Molecular Mass (kDa) | Functions |
| C | 21-22 | Nucleocapsid, RNA binding |
| 16, 19 | Unknown | |
| El | 31-35 | Envelope protein |
| E2 | 70-72 | Envelope protein |
| P7 | 7 | Unknown |
| NS2 | 21-23 | NS2-3 protease component |
| NS3 | 70 | NS3/4 protease (protease domain) |
| NS2/-3 protease (protease domain?) | ||
| NTPase | ||
| RNA relicase | ||
| RNA binding | ||
| NS4A | 8 | NS2/4 protease-cofactor |
| NS4B | 27 | NS5A phosphorylation |
| NS5A | 56-58 | Unknown |
| NS5B | 65-68 | RNA dependent RNA polymerase |
Features of HCV13
- Related to flavivirus and pestiviruses.
- Positive-strand RNA genome approximately 9400 nucleotides in length.
- Estimated size 30-50 nm
- Genomic hypervariability, particularly in nonstructural regions (51 UTR well conserved).
- Single open reading frame encoding a polypeptide of 3010-3011 aminoacids.
- Inactivation by chloroform, formalin, heat (100°C for 5 min, 60°C for 10 h), and beta-propiolactone-ultraviolet light.
- Pathogenic mechanisms unknown; presumed to be hepatocytopathic.
Geographical Distribution of HCV Genotypes
Regarding the heterogeneity of HCV at least six distinct genotypes and more than 80 subtypes within genotypes of HCV have been identified worldwide by nucleotide sequencing based on analysis of complete or partial genomic sequences. Because divergence of HCV isolates within a genotype or subtype, and within the same host, may vary insufficiently to define a distinct genotype, these intragenotypic differences are referred to as quasispecies. The genotypic and quasispecies diversity of HCV, resulting from its high mutation rate, interferes with effective humoral immunity8,72. Neutralizing antibodies to HCV have been demonstrated, but they tend to be short lived, and HCV infection has not been shown to induce lasting immunity against reinfection with different virus isolates or even the same virus isolate. Thus, neither heterologous nor homologous immunity appears to develop after acute HCV infection. Some HCV genotypes are distributed worldwide, while others are more geographically confined. In addition, differences in pathogenicity and responsiveness to antiviral therapy have been reported among genotypes.
Prevalence of genotypes vary from place to place. The most common genotypes in the United States and western Europe are la and lb14. Genotype Ib has been reported to be associated with higher HCV
HCV genotype in volunteer blood donors14
| HCV genotype | ||||||
1 | 2 | 3 | 4 | 5 | 6 | |
| Australia | 50% | 13% | 33% | 0 | 4% | 0 |
| Scotland | 47% | 14% | 39% | 0 | 0 | 0 |
| Egypt | 0 | 0 | 0 | 90% | 0 | 0 |
| Taiwan | 57% | 43% | 0 | 0 | 0 | 0 |
| Japan | 77% | 23% | 0 | 0 | 0 | 0 |
| Hong Kong | 59% | 3% | 0 | 0 | 0 | 32% |
RNA levels in the infected host, more advanced disease and suboptimal response to currently accepted therapy. Genotypes Ib, 2a and 2b are common in Japan and Taiwan; Genotype 3 has been described in Thailand, Northern Europe and Australia; genotype 4 is predominant in the Middle East; genotype 5 is prevalent in South Africa and genotype 6 has been reported in Hong Kong. HCV 3 is prevalent in India and HCV 6 in South East Asia10,14. The relationships between genotype and clinical manifestations, mode of transmission, hepatocellular carcinoma (HCC), viral load and response to treatment are still being elucidated. Multiple genotypes may infect a single individual, with one genotype being dominant at any particular time with immune selection pressures (possibly including antiviral therapy) other variants may become dominant14. In Australia it has been demonstrated that patients with genotype Ib have often emigrated from Mediterranean countries (92%), often have no parenteral risk factors (66%) and that initial and long-term response rates to interferon are inferior compared to type 3.
Quasispecies of HCV
As is characteristics of RNA viruses, HCV within infected hosts exhibits as a variable complex of related genomes, quasispecies there is a substantial fluidity of HCV genome resulting from an error prone replicase and absence of repair mechanisms that operate during DNA replication6‘15. This means that, even in a single infected individual, the HCV genome does not exist as a homogeneous species. Rather, it exists as a quasispecies distribution of closely related but nevertheless heterogeneous genomes (Marlell et al. 1992). In addition the process of host selection and adaptation of a rapidly mutating genome has led to the evolution of many distinct (yet still fluid) HCV genomes. Several different HCV genotypes can be distinguished according to the actual degree of nucleotide and amino acid relatedness and it is likely that many others will be covered in the future.
Hepatitis C virus is a positive-sense, single-stranded RNA virus and has been shown to be a major causative agent of non-A, non-B hepatitis. As is characteristic of RNA viruses, HCV within infected hosts exists as a variable complex of related genomes, quasispecies, generated by the lack of proofreading activity of RNA-dependent RNA polymerases. The quasispecies nature of HCV has been shown in the hypervariable region 1, the 5′ untranslated region, the core to envelope region, and the NS3 region. The quasispecies character has been reported to relate to the mechanism escaping from the host immune system, and also to be a predictive factor for interferon therapy15. Furthermore, the character may correlate with the progression of liver disease. Thus, studies on the quasispecies nature of HCV are important for understanding the-pathogenesis of chronic hepatitis C.
Features of the evolution of RNA virus quasispecies17
- Mutant genomes arise continuously at high rates.
- Negative selection (elimination of unfit mutants) is continuously acting during RNA genome replication.
- Mutant spectra are important reservoirs of genetic and phenotypic variants (antibody and cytotoxic T lymphocyte escape mutants, drug resistant mutants, cell tropism and host range mutants, virulent revertants of attenuated viruses, etc.).
- The types and numbers of mutants present in a mutant spectrum increase with the population size.
- Vital populations may maintain a relatively constant average or consensus sequence, but nevertheless include a dynamic and complex mutant spectrum.
- During natural evolution of virus periods of relative evolutionary stasis may alternate with periods of rapid change. The rate of accumulation of mutations is not constant and it generally varies different viral genomic regions.
Implications of quasispecies
Such heterogeneity has important implications in the developments of diagnostic assays. The quasispecies distribution of HCV genomes has major implication in various aspects of HCV infection and related disease. HCV quasispecies heterogeneity plays a, major role in viral persistence, which occurs in more than 80% of the cases17. Indeed, viral persistence is characterized by the continuous generation of quasispecies variants escaping selection pressures combining the immune responses of the host and complex interactions with host cellular proteins. It is likely that there is a compartmentalization and different cell tropisms of quasispecies variants, the role of which in the pathogenesis of HCV-related disease remains to be elucidated. Finally, HCV quasispecies heterogeneity plays a major role in HCV resistance to interferon alpha therapy. The genetic complexity of HCV quasispecies was indeed shown to be an independent predictor of a sustained virological response to a standard dose (3 megaunits administered three times per week) of interferon alpha. In addition, it has been suggested that the presence of viral proteins with different amino acid sequences in various proportions could regulate HCV replication and the inhibitory effect of IFN-induced pressures, therefore influencing HCV sensitivity to interferon therapy17. Virus-host interaction may vary depending on the infecting HCV genotype, which could lead to important differences in pathogenicity, and the progression of disease. The presence of the hypervariable region in the HCV genome may also affect vaccine development. Immune selection of viral escape mutants in the E2 hypervariable region may be important mechanism by which HCV evades immunoelimination and subsequently develops into chronic infection.
Epidemiologic Characteristics
The cloning of the hepatitis C virus and subsequent development of sensitive antibody tests for its diagnosis indicate that infection with HCV is a major health problem worldwide. Together with practices that have facilitated HCV viral transmission for several decades, global expansion of the human population has probably helped HCV viral spread during the last decades, as described for other RNA viral agents.
Although universal screening of blood donors in developed countries and improvements in infection control measures following Centers for Disease Control and Prevention guidelines have decreased significantly exposure to the virus, which in the younger generations is confined to high-risk groups such as drug addicts, the large reservoir of chronically infected individuals, the high evolutionary potential of the virus, the lack of routine screening of donated blood in many countries, and the use of traditional medicine and tattooing in some cultures strongly support the hypothesis that HCV is still spreading throughout the world.
Transmission mechanisms and groups at risk for HCV infection
Blood transfusion and intravenous drug use have been the predominant mode of transmission in Eastern and Western hemispheres. Groups dependent on human blood products have the highest prevalences. However, these obvious parenteral routes of HCV transmission account for only the 30% to 70% of anti-HCV positive
patients depending on the area. Other potential routes of transmission such as unapparent parenteral or permucosal exposures in the transmission of the virus (e.g., medical intervention, tattooing, acupuncture, and vertical, sexual, accidental needle prick, and household transmission) are also discussed.
Parenteral Transmission
The parenteral route of HCV transmission is responsible for one-third to two-third of hepatitis C cases and constitutes the most commonly recognized and best characterized transmission mechanism of HCV. Anti-HCV testing has largely confirmed that HCV is responsible for the vast majority of hepatitis cases in which transfusion of blood or blood components or obvious percutaneous exposure to blood is involved. The incidence of HCV in some risk groups directly depends on the baseline prevalence of HCV in the general population so that recipients of blood products obtained from a low prevalence area have a low incidence of infection.
Transfusion Recipients
Before the implementation of mandatory anti-HCV screening in 1990, there was a wide range of transfusion associated hepatitis C incidences in different geographic areas, ranging from 0.5% in England, 1.1% in Australia, 3% to 4% in the-United States, 7.7% in Japan, 11% in Spain, 12.5% in Taiwan to 13% in Greece. As expected, patients requiring multiple transfusions have high prevalences of HCV infection. Among more than 1,000 transfusion dependent Italian thalassemics, 80% had confirmed anti-HCV, as well as 47% of Egyptian thalassemic children and 75% of multitransfused patients in long-term remission from leukemia with evidence of liver disease.
Screening of blood donors for anti-HCV has practically eradicated TAH-C, so that transfusion of screened blood should no longer be considered a primary risk factor for HCV infection. Currently, the risk of acquiring HCV infection from transfusion of screened blood is extremely small. A recent study estimated that in the United States, the current risk of receiving blood from a donor in the window period of infectivity before seroconversion would be approximately 1/100,000. At least three such cases have been reported in Europe during the last 2 years.
Plasma Product Recipients
The prevalence of HCV among hemophiliacs correlates with the amount and type of product transfused. Virtually all hemophiliacs exposed to untreated commercial clotting factor concentrates have evidence of HCV Infection, whereas among those treated with cryoprecipitates, the rate was 66%. In contrast, hemophiliacs who have exclusively received appropriately inactivated coagulation components or single donor cryoprecipitate are generally anti-HCV negative. Despite the dramatic reduction in the risk of HCV transmission after implementation of efficient virucidal methods, the risk may not have been completely eliminated. Screening of plasma pools used to manufacture concentrates should eliminate this small residual risk.
Hemodialysis patients
Among patients on maintenance hemodialysis, prevalence of HCV infection averages 20%, although there are wide geographical variations ranging from less than 5% in northern Europe to 30% to 50% in Japan, Poland, Saudi Arabia, Taiwan, South America, and Egypt. Intermediate prevalences, between 5% and 30%, have been reported from the United States, India, Hong Kong, Western Europe, and Thailand. The high prevalence of HCV infection in hemodialysis patients has been attributed not only to the frequency of blood transfusion among these patients, but also to increasing years on dialysis, suggesting that HCV may be transmitted among patients in the dialysis until, probably as a result of poor infection control practices. Comparison of HCV spread between two dialysis units in southern Sweden revealed that in one unit, there was no evidence of spread within the unit, and that the prevalence of HCV was dependent on the status of the patients entering for treatment6. In the other unit, 36% of patients were infected during a 3-year period, including patients who had not received blood transfusions.
Organ Transplantation
Organ transplant recipients are at high risk of acquiring HCV infection. Infection in this setting can occur as a result of recurrence of HCV infection already present before transplantation, transfusion-associated transmission during transplantation, or the presence of HCV infection in the organ donor.
Antibody tests may underestimate the incidence of transmission and the prevalence of HCV infection among immunosuppressed organ recipients. Hence HCV RNA testing may be required to detect those patients who do not develop HCV antibodies6.
Sporadic Hepatitis
Acute hepatitis C with no apparent risk factor continues to occur8,10. The mechanism of transmission of sporadic hepatitis C cases is possibly a combination of intravenous drug use, which is not revealed in the history; inapparent or covert, nosocomial, percutaneous exposure, nonpercutaneous mechanisms, including sexual transmission; and perhaps as yet unidentified modes of virus dissemination. Recently cocaine snorting has been suggested as a significant risk factor for HCV infection when it involves sharing of blood contaminated straws.
Prevalence of HCV Infection Among Blood Donors and the General Population
Very low prevalences have been reported in the United Kingdom and Scandinavia, New Zealand, and in some areas of Japan. Low prevalences ranging from 0.15% to 0.5% have been described in the United States, Jamaica, Western Europe, and Australia. Moderate prevalences between 0.6% and 1% have been found in some areas of Southern Europe, Kenya, Thailand, and Russia. Prevalences between 1% and 1.5% have been reported from India, China, Cuba, and Ethiopia. High prevalences between 1.6% and 3.5% have been found in Japan, Indonesia, some areas of Russia, Brazil, and the Middle East. Extremely high prevalences have been reported in some parts of Cameroon (6.4%), and in Egypt (14%). The highest prevalence thus far reported was found in Cairo (26%).
Confirmed anti-HCV positive blood donors have a history of overt percutaneous exposure to blood in 30% to 75% of the cases, this antecedent being higher in areas with a lower prevalence of HCV infection. Other factors significantly associated with HCV infection include a prior history of major surgery, the use of nondisposable needles, a history of tuberculosis or prolonged hospitalization before 1970 in older people, and the sharing of straws for cocaine snorting, and certain customs in specific hyperendemic areas10. The use of paid instead of volunteer blood donations-has been implicated as the cause of very high prevalences of HCV in some hyperendemic areas. The lack of HCV infection, but not of hepatitis B, D, or E virus infection, among Amerindian populations traditionally excluded from any health care system further supports that health care-related covert parenteral exposures may have been an important mechanism of HCV spread in the developed world in the recent past. For instance, the indigenous tribe Parakana had no HCV infection during the first years in which they initiated outside contact in the 1970s and 1980s, but currently Parakana people have a prevalence of 1.4% to 1.6%.
Because the selection of regular volunteer blood donors generally excludes high-risk subjects for blood-borne infections or individuals with surrogate markers for such infections, the extent to which prevalences among blood donors can be extrapolated to the general population remains controversial, because: (1) prevalence among blood donors markedly increases with age and is slightly higher in men than in women; (2) confirmation of antibody specific in E1A reactive donors varies markedly from country to country, although it tends to parallel the prevalence of infection in the general population, so that less than 50% of EIA-reactive samples are confirmed in low-prevalence areas, as compared with more than 50% in areas of higher prevalence; (3) changes in blood donor recruitment policies may render data on first-time donors unreliable for extrapolation of prevalence to the general population; (4) prevalence studies in nondonor populations are scarce and usually limited to particular groups such as pregnant women or military recruits, which are biased by age and/or sex preselection or are restricted to small isolated peculiar populations; and (5) different geographical areas or specific populations within the same country may have widely different prevalence rates because of peculiar modes of covert percutaneous exposure.
The most important routes of HCV transmission have been parenteral, including administration of therapeutic blood products intravenous drug use, and nosocomial transmission from patient to patient as in hemodialysis, patient to health care worker after accidental needle prick, and health care worker to patient during surgery or other invasive procedures6,10,16.
World prevalences of HCV infection among blood donors.
County, Area | Prevalence (%) | Assay | Comments | Citation | |
| England Northern Ireland Norway New Zealand United States Italy Australia Jamaica Thailand Moscow Tuva, Russia China Mnenti Beijing Cuba India Japan Saudi Arabian Middle East donors Jeddah Hanoi HoChi MinhCity Egypt Egypt (Cairo) | 0.07 0.07 0.09 0.10 0.2-0.8 0.2 0.25 0.3-0.4 0.8 0.91 1.75-2.1 1.2 0.9 0.3 5.7 1.5 1.5 2.0 0.66 2.87 3.2 0.8 16 13.6-14.4 26.6 | RIBA-2 RIBA-2 RIBA-2 RIBA-2 ct RIBA-2 PCR RIBA-2 PCR RIBA-2 RIBA-2 RIBA-2 I’CK PCR PCR ct ct ct RIBA-2 RIBA-2 EIA-2 PCR PCR RIBA-2 RIBA-2 |
Voluntary donors Paid donors
1.7% Saudi 0.5% Bedouin 4.2% non-bedouin | Dowetal. Dow et al. Nordoy et al. Jackson et al. Alter, Davvson et al, Kleinman et al. Shakil et al. Marranconi et al. Allain et al. King et al. Luengrojanakul et al. lashina et al. lashina et al. llako et al. Audi oi al Wang et al. Galban Garcia et al. Irshad & Achar a Watanabe et al. Bernvil et al. Bernvil et al. Abdelal et al. Song et al. Darwish et al. Bassilv et al. | |
ct (confirmatory tests) include RIBA, RT-PCR, Ino-Lia.
Prevelence of anit-HCV in blood donors and in the general population from different geographical areas10.
% Anti-HCV positive Studies in blood donors | References | |
| Scandinavia | 0.01-0.1* | Mathiesen, Scan JGastr, 1993; 28:58l;Nordoy, ScajGasti. 1994; 29:77 |
| United Kingdom | 0.01-0.7* | Bow, JMedVirol, 1993:41:215; Conlon, IrJMedSci. 1993:162:142. |
| Greece | 0.7* | Hadziyannis, JHepatol, 1993; 17:572. |
| France | 0.3* | Ranger, Gut: 1933;34(Suppl.2):S50. |
| Italy | 0.8* | Lai, JMedVirol, 1993:41:282. |
| Crcatia | 0.7* | Mihaljevic. VoxSang. 1992:63:236. |
| Russia | 1.4-4.8* | Mikhailov, p. 175 in ref. 4. |
| United States | 0.5* | Kleinman. Transfusion, 1992:32:805. |
| Brazil | 2.9* | Patino Sarcinelli, Transfusion. 1994:34:138. |
| Australia | 0.06-0.31* | Archer, MedJAust, 992:157:225; National Health and Medical Research Council Report, 1993. |
| New Zealand | 0.1* | Jackson, NZMedJ, 1994; 107:10. |
| China, Macau | 0.9* | Aach, NtJM, 1991:325:1325. |
| Japan | 1.9* | Yano, Gut. l993;34(Suppl. 2)SI3. |
| Saudi Arabia | 3.2 | Abdelaal, Transfusion, 1994:34:135. |
| Egypt | 25 | Arthur, Ti’iennal, p. 178 in re I’. 4 |
| Ethioia | 1.4 | Frommel, AmJTropMedHyg, 1993:49:435. |
| France | 0.68* | Janot, Hepatol, 1995:21:725 |
| Studies in the general population | ||
| Italy | 3.2 | Bellentani, Hepatol, 1994:20:1442. |
| United States | 1.4 | Alter, SemLivDis, 1995:15:5. |
| Peru | 0 | Hyams. JMedVirol. 1992:37:127. |
| Africa | 0.6-6.5* | Frame, AmJTropMedHyg. 993:49:435; Delaporte, TrRSocTropMedHyg. 1993:87:6366; Abdool Karim. SAMedJ. I993;83:I9I. |
| Korea | 1 | Kirn, GastrJap, 1993:28:17. |
| Yemen | 2.6* | Scott, AmJTropMedHyg, 1992:46:63. |
| Tajwan | 5.6 | Chien, JGastrHepatol, 1993:8:574. |
| Japan | 5.1 | Tajima, Lancet, 1991:337:1410. |
| China | 2.1 | Tao, GastrJap. 1991:26:156. |
Data were confirmed by supplemental anti-HCV RIBA and / or HCV RNA polymerase china reaction testing.
Prevalence of anti-HCV in risk groups
Risk group | % anti-HCV positive | References |
| Thalassaemics | 42-83 | Ni, PediatKes, 1996:39:323; Rcsti. LurJPed. 1992:151:573. |
| Haemophillics | 50-95 | Kumar, JMedVirol. 1993:41:205: Jackson. NZMedJ. 1994:107:10. |
| Haemodialysis patients | 10-45 | At Nasser, VoxSang, 1992:62:94; Nordenfeldt. JMedVirol. 1993:40:266. |
| Health care professionals | 0-10 | Germanaud, mJPubllllth, 1994:122:84: Klein. Lancet. 1991:338:1539. |
| Intravenous drug abusers | 48-90 | Esteban, Lancet. 1989; ii:294; Verbaan. ScaJGastr. 1993:28:714. |
| Tattooed persons | 11 | Ko, JMedVirol, 1992:38:288. Kelen, NEJM. 1992:326:1399. |
| Patients with history of blood transfusion or major surgery | 21 | De Mercato. MinMed, 1995:86:89; Holsen. EurJCIinMicrob. 1993:12:673. |
| Prisoners | 15-46 | Kelen. NLJM. 1992:326:1399. |
| Patients admitted to emergency room | 8 | Klein, Lancet. 1991; 38:1539. |
| Dentists practicing oral surgery | 9 | Pereira, NEJM, 1992;327:910. |
| Recipients of transplanted organs from anti-HCV positive donors | 62 | Verbaan, ScaJGastr. I993;28:714: Zarski. JHepatol. 1993:17:1014. |
| Alcoholics | 15-25 | Pregliasco, EurJEpid. 1994:10:113, Cluing. MJedVirol. 1993:40:170. |
| Handicapped or institutionalized individuals | 4-7 | Everhart, AnnlntMed. 1990:112:54; Akahane. AnnlntMed. 1994:120:748. |
| Heterrosexual partners of HCV carriers | 0-18 | Hyams. JA1DS, 1993:6:1353; Lissen. EurJClinMlcrobiol, 1993:12:827. |
| Prostitutes | 0.7-6 | Thomas, JlnfDis. 1993:167:66; Quaranta. JMedVirol. 1994:42:29. |
| Household non-sexual contacts of HCV carriers | 3.18 | Deny, JHepatol. 1992:14:409; Ideo. Lancet. 1990:335:353: Diago. JHepatol. 1996:25:125. |
| Children of HCV-infected mothers | 0-11 | Ohio. NEJM, 1994:330:744; Lam. JlnfDis. 1993:167:572. |
Transmission routes for HCV infection10
Proved and highly efficient | Proved or highly Suspected but not very efficient | Suspected but not proved |
| Blood transfusions | Needle prick injury | Haemodialysis facilities |
| Intravenous drug abuse | Maternofetal or neonatal | Acupuncture |
| Organ transplantation | Sexual contact | Ear piercing |
| Household contact Tattooing | Intranasal cocaine use |
Seroepidemiology of HBV and HCV in Bangladesh18
Virus related liver diseases are important cause of morbidity and mortality in Bangladesh. HBV accounts for 35% acute viral hepatitis, 40.5% chronic liver disease, 36.5% HCC and 29.1% cases of post transfusion hepatitis. HCV accounts for 3.5% acute viral hepatitis, 24.1% chronic liver disease, 9.6% HCC and 6.8% cases of post transfusion hepatitis. 29% of professional blood donors and 2.4% of voluntary blood donors are HBsAg carriers. Anti-HCV was found in 1.2% of professional blood donors and in no voluntary donors. HBsAg is positive in 7.5% of healthy adult jobseekers. There was no history suggestive of parenteral route of infeciton in 60% cases of HBV and 54% cases of HCV. In another study during 1994-1996 43,213 Bangladesh job seekers were screened for HBsAg and serological tests showed that 1884 (4.4%) were positive for HBsAg25.
In conclusion, HBV is as yet the major aetiological factor for chronic liver disease and HCC. Whereas, HCV is emerging as the second important aetiological factor for such disease in Bangladesh.
Hepatitis virus markers in blood donors in Bangladesh and Japan20
| No. | HBsAg | Anti-HBs | Anti-HBs | Anti-HCV-2 | ||
| Bangladesh | ||||||
| Professional | 163 | 48(29) | 34/74(46) | 62/74(84) | 2(1.2) | |
| Voluntary | 83 | 2(2.4) | – | – | – | |
| Japan | ||||||
| Voluntary | 7479 | 43(0.6) | 822(11) | 973(13) | 47(0.6) |
These results indicates that professional blood donors in Bangladesh are highly contaminated by hepatitis B virus and moderately by hepatitis C virus20.
Viral marker positivity in the two groups of patients21
No | Age range (yrs) | No (%) male | Viral Marker Passivity | (%) | |||
HBSAg (HBeAg) | HB eAb (in HBSAg -Ve) | HCV Ab | HDV Ab | ||||
| Group 1 (n=46) | |||||||
| HCC | 16 | 30-75 | 14(88) | 38(60) | 17 | 56 | 0 |
| Cirrhosis | 10 | 24-62 | 7(70) | 40(50) | 30 | 30 | 0 |
| Chronic viral hepatitis | 20 | 20-65 | 16(80) | 30(83 | 45 | 50 | 0 |
| Group 2 (n=8) | |||||||
| HCC | 8 | 45-66 | 8(100) | 0 | Not done | 0 | Not done |
N.B.: Group Bangladeshi subject, Group 2 Bangladeshi subjects, resident in UK.
Recently in another study “Human immunodeficiency virus, hepatitis B, C and D in Bangladesh’s trucking industry: prevalence and risk factors” HCV prevalence has been studied among 388 (245 drivers and 143 helpers) individuals. This population was working out of Tejgaon truck stand in Dhaka. The prevalence of diseases was HIV 0%, HCV <1%, and HBsAg 5.922.
Prevalence and risk factors of hepatitis B virus, hepatitis C virus and HIV infections among drug addicts in Bangladesh were studied among 266 drug users attending drug addiction treatment centre in Dhaka, Bangladesh in 1997. The seroprevalence of HBsAg, Anti-HBc And HBs and And HCV – 8 (6.2%), 41 (31.8%), 15 (11.6%) and 32 (24.8%) respectively among IDUs and among non-lDUs (non injectable drug users) 6 (4.4%), 33 (21.1%), 9 (6.6%) and 8 (5.8%) respectively23.
Viral Hepatitis: recent experiences from serological studies in Bangladesh indicates the prevalence among patients suspected of having infection by HBV, HCV, HAV and HEV – Antibody to HAV 39%, HBsAg 19%, and HCV 13% and anti HEV 53%24.
Hepatitis B and hepatitis C virus study in patients suffering from End Stage Renal Failure on maintenance haemodialysis in Bangladesh has shown that 10% patients were positive for anti HCV and 2.5% were positive for HBsAg in a population of forty ESRD patients on maintenance hemodialysis82.
Transmission of Hepatitis C Virus Transmission
Early studies in transfusion recipients demonstrated that HCV is efficiently transmitted by blood and blood products. However, in the various studies only 5 to 35 per cent of patients with hepatitis C had been transfused, a percentage that has much declined after introduction of anti-HCV screening of blood donors. A major contribution to the pool of acute and chronic HCV infections is provided by intravenous drug abusers, who account for up to 50 per cent of infections in some geographical areas. Overall, other identified parenteral routes accounted for no more than 5 per cent of exposures in the Centers for Disease Control study of community acquired, non-A, non-B hepatitis. Therefore a consistent proportion of patients with hepatitis C have no identifiable percutaneous exposure. The source of infection and the modes of HCV transmission in this setting remain a matter of debate and concern.
Data from the Centers for Disease Control and Prevention indicate that between 1991 and 1995 about 30 per cent of patients presenting with acute hepatitis C had no definite risk factors for parenteral exposure during the 6 months preceding onset of the disease, although in many of them detailed evaluation of past history indicated at risk behaviours26.
Non-transfusional percutaneous transmission
The importance of percutaneous transmission outside the transfusional setting is emphasized by the prominent role of hepatitis C as the cause of acute and chronic liver disease in patients who have never been transfused. Intravenous drug abusers represent a consistent reservoir of HCV infection. The main route of HCV transmission in this setting is the sharing of contaminated needles. Recently, sharing of straws has been reported as a potential route of transmucosal transmission in nasal cocaine users. Drug abusers who started to self-inflict before the AIDS prevention campaigns and the discovery of HCV have anti-HCV prevalence rates greater than 90 percent; most have evidence of liver disease. Although in some areas the prevalence of anti-HCV in drug abusers has diminished in the 1990s, new infections continue to occur in this setting; with the dramatic reduction of transfusion associated hepatitis, hepatitis C occurring in drug abusers accounts at present for 40 to 50 percent of all cases of acute hepatitis C10,26.
Transmission from patient to patient, possibly through medical procedures, may be responsible for nosocomial outbreaks of parenterally transmitted hepatitis C on haematology and oncology wards27.
Covert non-percutaneous transmission
A large percentage of patients with acute or chronic hepatitis C have, no history of an identifiable parenteral exposure; household, sexual, or maternal-infant transmission is suspected.
A number of studies have raised the possibility of sexual transmission of HCV. A CDC study involving patients with non-A, non-B hepatitis and non-infected controls showed that patients with hepatitis were more likely than the general population to have a history of unprotected sexual contact with a person with hepatitis and multiple sexual partners. After the discovery of HCV, higher infection rates have been reported among sexually exposed individuals than in the general population. Prevalence rates among heterosexual partners of infected individuals average no more than 5 percent in Western countries.
However, a Japanese study of 154 spouses of patients with index cases of hepatitis C reported that 18 per cent were HCV RNA positive. Genotype analysis showed that 89 per cent of these spouses had the same genotype as their partners, confirming partner-to-partner transmission. Sexual transmission of HCV seems enhanced when the partner is coinfected with HIV; this could be related to the high levels of HCV viraemia induced by HIV associated immunodeficiency. The prevalence rate of HCV infection in male homosexuals is somewhat higher than in the general population, but remains well below the prevalence rates for HBV and HIV infections in the same risk group; no correlation was found between the prevalence of anti-HCV and sexual practices that are thought to enhance viral transmission. The rate of HCV infection is higher among male homosexuals with HIV infection; the same is true for prostitutes, clients of prostitutes, and individuals attending clinics for sexually transmitted diseases.
The question of perinatal transmission of HCV has been addressed by many investigators. The diagnosis of perinatal infection is made from the persistence of anti-HCV in the serum for more than 12 months after birth and/or on the detection of HCV RNA in at least two post-delivery samples; however, the antibody based diagnosis may not be reliable as in a few eases passively transmitted antibodies may persist for longer than 12 months. Even with stringent diagnostic criteria, however, most epidemiological studies show minimal evidence for mother to infant transmission, the average the being 5 per cent. Factors that influence vertical transmission appear to be the HCV RNA titre in the mother and maternal co infection with HCV and HIV. The analysis of HCV RNA titres in mothers positive for anti-HCV indicates that only those with HCV RNA titres higher than 106 transmit infection to their babies; transmission from highly viraemic mothers occurs in 36 per cent of newborn infants. Two studies in urban areas of northern Italy have shown a high rate of perinatal transmission from drug abusing mothers coinfected with HCV and HIV, compared with no infection in babies been to mothers circulating HCV alone; however, these data were not confirmed10.
As the overall efficiency of sexual, household, or perinatal routes of transmission of HCV seems to be low, the transmission modes of HCV in the community remain controversial. Surreptitious parenteral drug abuse, overlooked transfusions received in infancy, covert routes by which blood may be transported from individual to individual such as acupuncture, tattooing, sharing of tooth brushes and razors, ear piercing, and other means of traumatic contact with objects contaminated with blood have been advocated. Potential exposures such as sexual and household contacts, though of limited transmission efficiency, may occur so frequently as to account cumulatively for large numbers of infected persons.
Various methods of transmission of HCV
- Transmission by intravenous drug use.
- Transmission by blood and blood products.
- Transmission by organ and tissue transplantation.
- Transmission by undefined routes (“Sporadic” or community acquired” with no readily identifiable risk factors).
- Transmission to non-sexual close contacts.
- Transmission through tattoos.
- Sexual transmission.
- Vertical spread (Mother to infant transmission).
- Occupational exposure to blood and blood products.
- Nosocomial transmission.
- Transmission through non-human vectors.
- Transmission to contacts of individuals infected by one of the other routes.
Pathogenesis
HCV is an RNA virus that belongs to the family of flaviviruses; the most closely related human viruses are hepatitis G virus, yellow fever virus, and dengue virus. The natural targets of HCV are hepatocytes and, possibly, B lymphocytes29. Viral replication is extremely robust, and it is estimated that more than 10 trillion virion particles are produced per day, even in the chronic phase of infection. Replication occurs through an RNA dependent RNA polymerase that lacks a “proofreading” function, which results in the rapid evolution of diverse but related quasispecies within an infected person and presents a major challenge with respect to immune-mediated control of HCV.
Despite in vivo replication rates in excess of those observed in HIV-1 and HBV infection, efforts to grow HCV in culture have been largely unsuccessful. Injection of recombinant transcribed HCV RNA into chimpanzees has resulted in the successful propagation of virus, accompanied by clinical and histologic signs of hepatitis. Recent genetic manipulations of the RNA of virions have resulted in high-level replication in cell lines derived from hepatocytes, offering a more tractable means to study viral RNA and protein synthesis30.
HCV encodes a single polyprotein of 3011 amino acids, which is then processed into 10 mature structural and regulatory proteins. Structural components include the core and two envelope proteins. Two regions of the envelope E2 protein, designated hypervariable regions 1 and 2, have an extremely high rate of mutation, believed to be the result of selective pressure by virus specific antibodies. E2 also contains the binding site for CD81, a tetraspanin expressed on hepatocytes and B lymphocytes that is thought to function as a cellular receptor or coreceptor for the virus. HCV also encodes a virus specific helicase, protease, and polymerase, and because of the critical function of these proteins in the viral life cycle, they represent attractive targets for antiviral therapy. Similarly, the untranslated regions at both ends of the viral RNA may show promise as therapeutic targets, since they are highly conserved and involved in critical stages of viral replication.
Six distinct but related HCV genotypes and multiple subtypes have been identified on the basis of molecular relatedness. In the United States and western Europe genotypes la and Ib are most common, followed by genotypes 2 and 3. The other genotypes are virtually never found in these countries but are common in other areas, such as Egypt in the case of genotype 4, South Africa in the case of genotype 5, and Southeast Asia in the case of genotype 6. Knowledge of the genotype is important because it has predictive value in terms of the responses to antiviral therapy, with better responses associated with genotypes 2 and 3 than with genotype 1. Certain strains of HCV may have enhanced virulence, although the specific molecular determinants that may confer this phenotype have not yet been identified. Variability within a region of the gene for nonstructural protein 5 appears to have particular clinical significance in determining the sensitivity to interferon, as shown in isolates of Japanese subtype Ib. However, European and American isolates of HCV Ib do not share this property to the same degree31.
In most persons who become infected with HCV, viremia persists, accompanied by variable degrees of hepatic inflammation and fibrosis. Earlier studies of chronic HCV infection suggested that only a small number of hepatocytes become infected, but more recent estimates suggest that 50 percent or more harbor the virus31.
The presence of lymphocytes within the hepatic parenchyma has been interpreted as evidence of immune mediated damage. Recent studies of acute HCV infection in chimpanzees and humans, however, suggest that immune-mediated control of HCV may be possible. Viral clearance is associated with the development and persistence of strong, virus-specific responses by cytotoxic T lymphocytes and helper T cells. The responses of helper T cells appear to be critical, since the loss of these cells has been linked to the reemergence of viremia. The finding that viral diversity is reduced in persons in whom the infection is cleared is also consistent with the occurrence of greater immune mediated control of the virus32.
Two potential mechanisms may be responsible for the damage of viral infected cells in vivo, direct cytopathicity and immune mediated injury, either targeting the virus or an autoantigens6,28. Direct cytopathic effects are usually the results of the toxic actions of virion components or virus-specific products. Viruses can also interfere with the synthesis of cellular macromolecules, increase lysosomal permeability, and alter cellular membranes. Direct cytopathicity is usually recognized by morphological alterations of cellular architecture28.
Immune-mediated mechanisms rely on the lysis of viral-infected cells by either direct lymphocyte cytotoxicity, antibodies or viral-induced auto-immune phenomena28. For viral specific lymphocyte cytotoxicity, antigen-presenting cells (APC) recognize and phagocytize viral particles. After processing, viral proteins are presented to helper/ inducer T-lymphocytes (CD4+). The helper/inducer T-lymphocytes in turn, activate suppressor/cytotoxic. T-cells (CD8+) which attack target cells expressing processed viral peptides in conjunction with the human leukocyte antigen (HLA) class 1 molecules. For antibody mediated injury, APC present the viral antigen to B-cells and induce the production of specific anti-viral antibodies which mediate the elimination of viral infected cells either through complement cascade and/or antibody dependent cellular cytotoxicity.
Direct cytotoxicity
Cell damage in flavivirus infection has been attributed to direct cytopathic effects on target organs. In experiment of flaviviral infections in monkeys, cell damage is seen with minimal inflammatory response28. Yellow fever virus (a flavivirus) also cause severe hepatocellular damage without prominent histologic inflammation. The virion induced cellular alterations include cell rounding, shrinkage and nuclear pyknosis. At the ultramicroscopic levels damage to the mitochondria and endoplasmic reticulum, formation of inclusion bodies and cytoplasmic rarefaction have been reported. Early histologic studies of liver sections from patients with parenterally acquired chronic NANB hepatitis also suggested that the NANB agent was likely to induce liver cell by direct cytopathicity since some of the aforementioned features were observed. Theoretically, if intracellular viral accumulation is responsible for direct cytopathicity, there should be a correlation between hepatocellular damage and hepatic viral load. Indeed, in a recent study of 23 patients with chronic hepatitis C, the hepatic, but not the serum, HCV RNA levels correlated with more severe histologic inflammation. Another study found a correlation between HCV viraemia and lobular inflammation. In addition, interferon therapy was shown to reduce serum HCV RNA and this was paralleled by a decline in aminotransferase levels.
Immune-mediated mechanisms
Even though flaviviruses are believed to induce cell damage by direct cytotoxicity, both structural and nonstructural proteins of dengue virus (a flavivirus) have been identified on the surface of infected cells by immune electron microscopy, and are thus susceptible to immune mediated lysis28. There are several other lines of evidence which suggest that immune mediated mechanisms may also play a role in the pathogenesis of hepatocellular damage in chronic HCV infection28.
Firstly, intraportal lymphoid aggregates are commonly observed in patients with chronic hepatitis C. In one study, the presence of intraportal lymphoid aggregates was associated with more severe liver disease. These aggregates consists of a germinal centre with activated B-lymphocytes containing helper cytotoxic/ suppressor and activated T-lymphocytes. The prominent intrahepatic lymphocytes in chronic NANB hepatitis are CD8+, suggesting that cytotoxic T cells might be involved in the mediation of liver damage. Furthermore, no significant differences was observed between the lymphocyte subsets in chronic NANB hepatitis and chronic hepatitis B, a disease whose immunopathogenesis is established. Interestingly, the hepatic expression of activation markers including interleukin-2 and transferrin receptors was poor but a large proportion of intrahepatic CD8+ cells expressed other activation markers such as T11/3 and “activation-inducer molecule”. These findings raise the possibility that some hepatic CD lymphocytes may be activated by an alternative antigen independent pathway.
Secondly, co-culture experiments of peripheral blood lymphocytes with autologous hepatocytes, which satisfied HLA restriction, demonstrated significant hepatocyte toxicity in patients with chronic NANB hepatitis, suggesting the presence of a cellular immune mechanism for the elimination of viral infected cells. No cytotoxic effect was observed when lymphocytes from patients with NANB hepatitis were cultured with allogenic hepatocytes (not HLA restricted) or hepatitis B virus infected hepatocytes, providing further evidence for the specificity of the autologous lymphocyte cytotoxicity assay. The cytotoxicity observed in NANB hepatitis was mainly effected by T-lymphocytes. In contrast, the immune effector arm was restricted to non-T lymphocytes in autoimmune hepatitis and both non-T & T-lymphocytes were responsible for liver injury in chronic HBV infection. The role of T-lymphocytes in chronic NANB hepatitis was further substantiated by the establishment of a human T-cell clone capable of lysing autologous and allogenic hepatocytes. This clone expressed CD3, CD8 and CD56, which is a marker of natural killer cells. Thus, it was not surprising that the observed cytotoxicity was not HLA class I restricted. Recently CD8+, CD56+, T-lymphocytes that were activated by specific HCV antigens were demonstrated, which further supports the hypothesis that CD58+ lymphocytes may be important in the pathogenesis of chronic hepatitis C. Proliferation assays also demonstrated the presence of circulating CD4+ lymphocytes that responded to recombinant HCV proteins in a large proportion of patients with chronic HCV infection. Interestingly, a greater lymphoproliferative response was noted in asymptomatic patients (“healthy carriers”), indicating that these CD4+ cells may be protective rather than mediating cell damage.
Autoimmune mechanisms
Early studies using the first generation enzyme immunoassay for anti-HCV (anti-C 100-3) linked HCV infection to type 2 autoimmune hepatitis, which is characterized by the presence of liver kidney microsomal autoantibodies (anti-LKM) in serum. Although the validity of this finding was challenged, it was subsequently confirmed using recombinant immunoblot assay (RIBA) for anti-HCV antibodies and PCR for the detection of HCV RNA. The incidence of HCV infection in patients with type 2 auto-immune hepatitis varies from 44% to 80% in studies from Italy, Spain and France. The exact relationship between HCV infection and this type of autoimmunity is unknown but it was recently shown that the majority of patients with HCV infection and anti-LKM also had circulating antibodies to a human derived epitope GOR28.
Whether the host’s antibodies, targeted against HCV antigens or HCV induced autoantigens/neoantigens, mediate hepatocellular damage is still largely unknown. This question was addressed in one study and only 2 of 16 patients studied showed hepatocyte surface bound iminunoglobulin G(IgG) by immunofluorcscence. In contrast, liver cell membrane bound IgG is commonly found in chronic HBV infection and auto-immune hepatitis. Further studies are needed to elucidate the relationship between expression of HCV and HCV related antigens such as GOR, and liver cell surface bound IgG in order to better define its role in the pathogenesis of liver damage. In conclusions, the available information suggests that the HCV may induce hepatocellular damage by both direct cytopathicity and immune-mediated mechanisms.
HCV and hepatocellular carcinoma
HCV markers are frequently detected in patients with hepatocellular carcinoma, although with significant geographical variations. The highest rates of association are found in Southern Europe and Japan intermediate rates in Austria, Taiwan, and Saudi Arabia, and low rates in the United States, South Africa and in some countries of the Far East. Patients positive for anti-HCV with hepatocellular carcinoma almost invariably have an underlying cirrhosis, but there have been occasional reports of patients with hepatocellular carcinoma in a non-cirrhotic liver, and HCV has been implicated in a tumour arising in a normal liver. Sequence of HCV RNA are detectable in the tumorous tissue and virus replication has also been demonstrated within this tissue. The mechanism by which HCV infection promotes the development of hepatocellular carcinoma is not understood. The effect of the virus may only be indirect, causing cirrhosis which in itself is a risk factor for the development of this tumour; a direct oncogenic effect of HCV and/or virus products cannot be excluded10,28.
The risk for an individual chronically infected with HCV to develop hepatocellular carcinoma was assessed in retrospective and prospective studies. Sequential transition from acute hepatitis C to chronic hepatitis, cirrhosis, and hepatocellular carcinoma in individual patients has been well documented, with a time between infection and the appearance of the tumour of 5 to 30 years. The annual risk of developing hepatocellular carcinoma is around 0.5 to 1.5 percent for patients with chronic hepatitis C without cirrhosis and between 3 and 10 percent for patients with compensated HCV cirrhosis; it is higher in Japanese patients than in Caucasians. The continuing biochemical activity of liver disease increases the risk of developing this tumour. Coinfection with HBV and alcohol abuse is associated with an increased risk of hepatocellular carcinoma in those with cirrhosis.
Persistence of HCVUnlike HBV infection persistent HCV infection is not related to integration into the host genome because there are no DNA intermediates in the viral life cycle. Evidence of HCV replications, based on the presence of negative strand intermediates, has been documented in the liver but remains largely unsubstantiated in extrahepatic sites, such as peripheral blood mononuclear cells and serum. Persistence appears to result from the ability of the virus to replicate with a high rate of mutation, resulting in a series of immunologically distinct variants or quasispecies that allow the virus to escape immunologic control. Neutralizing antiviron antibodies develop but are isolate-specific and change over time; this may explain why both chimpanzees and humans can be reinfected with the same or different strains of HCV. An extremely high rate of mutation has been described in the HVR1 (E2HV) domain of HCV. Although the exact function of this domain has not been identified, mutations in this region of the envelope protein have been shown to contribute to the maintenance of HCV escape variants in persons with chronic infection.
Natural History And Clinical Course
At the time of the discovery of hepatitis C virus (HCV), there had already been much information on non-A, non-B hepatitis, particularly on the frequent evolution of chronic liver disease after acute infection. Soon, it was found that the vast majority, if not all, of NANB hepatitis cases in the past in fact had HCV infection when stored sera were analyzed, and that the information gained with NANB hepatitis was applicable directly to hepatitis C. HCV infection is worldwide, and the mode of transmission is somewhat different from that of hepatitis B virus. Persistent chronicity of HCV disease is now explained by the ability of this RNA virus to undergo genomic sequence changes for escaping from the host immune reaction. The epidemiology suggests certain regional differences in the distribution of genotypes of HCV, and in natural history, particularly the frequency of hepatocellular carcinoma as a sequela to chronic C disease. However, there are still a number of questions that have not been resolved regarding the clinical characteristics of hepatitis C, such as the prognosis of asymptomatic virus carriers, whether there is such a state as healthy carrier, whether treatment of chronic hepatitis C prevents hepatocarcinogenesis, how coinfection with HBV and hepatitis G virus modifies the clinical course, etc.
HCV infection is infrequently diagnosed during the acute phase of infection. Clinical manifestations can occur, usually within 7 to 8 weeks after exposure of HCV, but the majority of persons have either no
symptoms or only mild symptoms. Fulminant hepatitis has been described during this period, though it is very rare. In cases in which symptoms of acute hepatitis have been documented, they usually consisted of jaundice, malaise, and nausea. The infection becomes chronic in most cases, and chronic infection is typically characterized by a prolonged period in which there are no symptoms. An estimated 74 to 86 percent of persons will have persistent viremia, and this range may prove to be low as more sensitive tests become available to detect viremia.
The natural history of HCV infection has been very difficult to assess, because of the usually silent onset of the acute phase as well as the frequent paucity of symptoms during the early stages of chronic infection. Since the interval between infection and the development of cirrhosis can exceed 30 years, few prospective studies have been performed. Still, the data from retrospective and prospective studies allow several conclusions to be made. Acute infection leads to chronic infection in the majority of persons and spontaneous clearance of viremia once chronic infection has been established is rare. Most chronic infections will lead to hepatitis and to some degree of fibrosis, which may be accompanied by relatively nonspecific symptoms such as fatigue. Severe complications and death usually occur only in persons with cirrhosis, which is estimated to develop in 15 to 20 percent of those infected.
Two studies in women who received anti-D immune globulin contaminated by HCV in the late 1970s showed that after 17 to 20 years, more than 95 percent of those who had a liver biopsy had evidence of hepatic inflammation, but in most it was slight or moderate. Half had fibrosis, with only 2 percent having cirrhosis and 3 to 15 percent precirrhotic bridging fibrosis. Although these findings may be generally reassuring for the majority of infected persons, the high prevalence of the disease still translates into a large number of persons with clinical sequelae of disease. In addition, these figures may be an underestimate, because of the high percentage of favourable factors in the cohorts studied and the short duration of follow up. Furthermore, these studies concentrated on mortality and serious complications, but HCV infection can also have adverse effects on the quality of life even in the absence of severe disease33.
The time frame in which the various stages of liver disease develop is highly variable, with serious liver disease developing in one third of persons 20 years or less after infection and no progression in another third for 30 years or longer. Factors that accelerate clinical progression include alcohol intake, which has a pronounced effect on the course of the disease; coninfection with H1V-1 or HBV; male sex; and an older age at infection. Once cirrhosis is established, the risk of hepatocellular carcinoma is approximately 1 to 4 percent per year. Hepatocellular carcinoma can occur without cirrhosis but is rare33.
In addition to hepatic disease, there are important extrahepatic manifestations of HCV infection. Most of these syndromes are associated with autoimmune or lymphoproliferative states and may be related to the possibility that HCV is able to replicate in lymphoid cells.
Cryoglobulins can be found in up to half of persons with HCV infection, and the cryoprecipitates usually contain large amounts of HCV antigens and antibodies. Only a small fraction of affected persons (10 to 15 percent) have symptomatic disease. These symptoms are often related to vasculitis and consist of weakness, arthralgias, and purpura. The most severe cases are associated with membranoproliferative glomerulonephritis, as well as involvement of the nerves and brain. HCV is the chief cause of essential mixed cryoglobulinemia (type II cryoglobulinemia), with up to 90 percent of affected persons having HCV viremia. Since false negative tests for HCV antibodies are common in these persons, an HCV RNA assay should be used for diagnosis. A higher incidence of non-Hodgkin’s lymphoma has also been observed in HCV infection, both with and without mixed cryoglobulinemia. This correlation is not seen in all geographic areas; whether this difference is due to viral or host factors is not known. Other diseases, including lichen planus, sicca syndrome, and porphyria cutanea tarda, have been linked to HCV infection. However, a clear pathophysiological role of HCV has been difficult to establish.
Other clinically important syndromes include coinfections with other viruses, especially HIV-1 and other hepatitis viruses. In a large European cohort, 33 percent of HIV-1 positive patients were coinfected with HCV, and this percentage rose to 75 percent when the analysis was limited to patients with known injection drug use. With better treatment options for HIV-1, patients who are coinfected with HCV and HIV-1 will become an especially important group, since the course of HCV infection is accelerated by coinfection with HIV-1. After 15 years, the risk of cirrhosis in such patients was 25 percent, as compar
cirrhosis in such patients was 25 percent, as compared with 6.5 percent in those with HCV infection alone. Patients who are coinfected with HBV and HCV also have an accelerated course of disease.
Superinfection with hepatitis A virus (HAV) in persons who are infected with HCV can result in severe acute or even fulminant hepatitis. Vaccination of patients with HCV infection against HAV appears to be both safe and effective. Vaccination is recommended in these patients, as it is for other patients with chronic liver disease, although this approach is not cost effective in areas with a low incidence of HAV infection33.
In the majority of patients who contract acute hepatitis C, whether through blood transfusion, drug injection, or an unknown route (community acquired), the disease evolves into chronic hepatitis C. Spontaneous resolution of chronic hepatitis is uncommon, and chronic hepatitis C frequently progresses to cirrhosis and HCC. Disease progression is usually slow; it takes an average of 20 years to progress to cirrhosis from infection, and another 10 years from cirrhosis to HCC. HCC develops during active inflammation of the liver with elevated ALT and HCV RNA levels. The rate of HCC development among patients with chronic advanced C disease varies a great deal among countries. Of the various factors assumed to be involved in such differences, genotype of the virus seems to be significant. In Japan, Italy, and Spain, the annual rate of HCC development among patients with cirrhosis associated with HCV infection ranges from 3% to 6.5% a year, whereas cancer evolution seems much less common in other Western countries. Both coinfection with HBV and alcohol abuse aggravate the course of chronic C disease. The molecular mechanism that links the HCV genome and hepatocarcinogenesis is still poorly understood.
The rate of development of hepatocellular carcinoma in patients with cirrhosis6.
Author | Country | Reference | Year | No. Studied | Annual Rate (%) |
| Oka | Japan | 55 | 1990 | 140 | 6.5 |
| Colombo | Italy | 15 | 1991 | ‘447 | 3.2 |
| Ikeda | Japan | 30 | 1993 | 795 | 3. 9-4.4 (for C alone, %) |
| Sato | Japan | 66 | 1993 | 361 | 3.0 |
| Pateron | France | 59 | 1994 | 118 | 5.8 |
| Cottone | Italy | 16 | 1994 | 147 | 4.4 |
A. Progression rates from acute HCV infection to cirrhosis10
Progression (%) | Mean follow up (years) | |
| Tong, NEJM, I995;332:1463 | 50 | 21 |
| Di Bisceglie, Hepatol, 1991;14:696 | 24 | 23 |
| Koretz, AnnlntMed, 1993; 11 9: 110 | 18 | 16 |
| Wiese, JHepatol, 1995;23:89 | 0 | 15 |
| Tremolada, JHepatol, 1992; 16:273 | 32 | 5 |
| Booth, Gut, 1995;366:427. | 30 | 10 |
B. Histological progression in patients with chronic hepatitis C10
(1) | (2) | (3) | (4) | (5) | (6) | (7) | |
| CPH-remission | 4 | – | – | – | – | – | – |
| CPH-CAH | 4 | – | 4 | – | 4 | – | – |
| CPH-cirrhosis | 0.6 | – | 0.9 | – | 0.9 | <1 | – |
| CAH-remission | 0 | – | – | – | – | – | – |
| CAH-cirrhosis | 23 | 4 | 1.2 | 8 | 2-10 | 5 | 3 |
C. Liver disease related mortality in patients with post transfusion hepatitis C10.
Author | Follow up after transfusion (yrs) | % Death due to liver disease |
| Tremolada, J HepatoI, 1992; 16:273 | 10 | 4.8 |
| Tong, NEJM, 1995;332:1463 | 28 | 15 |
| Seeff, NEJM, 1992; 327:1905 | 18 | 3.3 |
| Di Bisceglie, Hepatol, 1991; 14:696 | 23 | 4-9 |
| Sanchez-Tapias (unpublished) | 10 | 3-6 |
Variables influencing the severity and course of chronic hepatitis C10.
| Variable | Type of association | Available evidence |
| Patient’s age | Stage of disease | Consistent |
| Duration of infection | Stage of disease | Consistent |
| Mode of transmission | Post-transfusion more severe than others | Controversial |
| ALT levels and profile | No association with histology | Consistent |
| Liver histology | Predictive of outcome | Mostly consistent |
| Viraemia levels | Activity and outcome of liver disease | Mostly consistent |
| HCV genotype | Activity and outcome of liver disease | Controversial |
| HCV quasispecies | Activity and outcome of liver disease | Controversial |
| HBV infection | Activity and outcome of liver disease | Consistent |
| Alcohol abuse | Activity and outcome of liver disease | Mostly consistent |
Extrahepatic manifestations
| Essential mixed cryoglobulinaemia | Lichen planus |
| Vasculitis | Sjogren’s syndrome |
| Membranoproliferative glomerulonephritis | Idiopathic pulmonary fibrosis |
| Porphyria cutanea tarda | Aplastic anaemia |
| Low-grade malignant non-Hodgkin a lymphoma | Polyarteritis nodosa |
| Mooren’s corneal ulcers | Erythema nodosum |
| Autoimmune thyroiditis |
Clinical features of chronic hepatitis C10
Clinical features at presentation | Post-transfusion hepatitis (%) | Community acquired hepatitis (%) |
Symptoms of disease | 39 | 25 |
| Jaundice | 12 | 0 |
| Hepatomegaly | 25 | 10 |
| Splenomegaly | 13 | 10 |
| Spidernaevi | 12 | 0 |
| Portal hypertension | 11 | 0 |
Biochemical features | ||
| ALT fluctuations | 90 | 75 |
| Raised IgG | 35 | 15 |
| Raised IgM | 0 | 0 |
| Raised-glutamyl transverse | 90 | 55 |
Histological features | ||
| Chronic persistent hepatitis | 20 | 45 |
| Chronic obular hepatitis | 22 | 25 |
| Chronic active hepatitis | 46 | 30 |
| Active cirrhosis | 12 | 0 |
Diagnostic Test
Diagnostic tests for HCV infection arc divided into serologic assays for antibodies and molecular tests for viral particles. Infection with HCV can be identified and staged with a variety of diagnostic tools that include tests for antiviral antibodies (anti-HCV) of the IgG and IgM type, direct detection and quantification of the virus genome (HCV RNA) in serum and in infected tissue and cells, and characterization of the genomic sequences of the virus to define the genotype and subtype of the infecting HCV.
Anti-HCV
Tests for serum antibodies against a variety of non-structural and structural HCV antigens are currently used to screen for hepatitis C and to diagnose past and present infection. Commercially available anti-HCV ELISA were initially based on the use of the C 100-3 epitope from the non-structural NS4 region of HCV. This test had a major impact on the screening of blood donors, leading to a drastic reduction of post-transfusion hepatitis C. However, it had low sensitivity in detecting hepatitis C and poor specificity in low-risk groups and in the presence of hypergammaglobulinaemia. Second generation ELISA were then developed which incorporated multiple viral antigens derived from the NS4 region of HCV (C 100-3 epitope) as well as from the NS3 region (C 33c epitope) and the structural core region (C 22-3 epitope). More recently third generation ELISA has been licensed, with the inclusion of antigen from the NS5 region and the C 22c and C 100-3 antigens as synthetic peptides. New generation assays have increased sensitivity compaired with first generation ELISA.
The diagnostic-reliability of anti-HCV ELISA tests varies in different populations. Hepatitis C may not be identified by HCV ELISA in patients who are in early phase of infection before seroconversion, in immunosuppressed or immunocompromised patients, including those who have received a transplant. In these patients hepatitis C can be diagnosed only with viral assays for serum HCV RNA. A positive anti-HCV ELISA test is diagnostic for hepatitis in high risk groups, namely individuals with a well-recognized risk for parenteral contamination, as well as in patients with acute or chronic liver disease and no other etiological factors, in these cases the use of supplemental anti-HCV tests is not recommended, as it would not be cost effective. However, up to 50 percent of anti-HCV ELISA reactivities may be false positive in low-risk individuals, namely those without parenteral exposure or evidence of liver disease10. False positive reactions are also seen in patient with hypergammaglobulinaemia, autoimmune reactions and diseases such as primary biliary cirrhosis and systemic lupus erythematosus, alcoholic liver disease, and metabolic disorders, as well as in pregnant women. Improper serum storage increases the risk of false reactions. The introduction of third generation assays has only partially solved the problems of specificity and in doubtful cases it is essential to use supplemental anti-HCV assays to confirm the diagnosis. The most commonly used assay of this type is the recombinant immunoblotting assay, but similar assays are also available. These tests allow visualization of individual antibody reactions against the same non structural and structural HCV antigens used in the ELISA tests. The antigens are spotted on to nitrocellulose stops and the reaction is recognized as a band in a different position for each antigen. Sera are defined as positive when they react with at least two viral antigens, in determinate when they react with a single viral antigen, and negative then no band of reaction with HCV antigens is seen.
Up to 30 to 50 per cent of sera positive for anti-HCV ELISA from low-risk individuals screened without a specific diagnostic indication are negative for anti-HCV RIBA and for HCV RNA by PCR, 20 to 30 per cent are determinate, and only 30 to 40 per cent are positive ELISA positive, RIBA negative results should be considered as non-specific reactions in the test system and not indicative of true HCV infection. These cases do not need further assessment or follow up. RIBA positive and indeterminate sera should be tested for serum HCV RNA to assess the phase of HCV infection. RIBA positive individuals often have HCV RNA in their serum and 80 to 90 percent transmit HCV infection via their blood. On the other hand, most RIBA-indeterminate reaction are non-specific and do not indicate ongoing HCV infection. However, about 25 per cent of symptomless subjects with an assistant indeterminate RIBA pattern may result positive for serum HCV RNA, particularly when the isolated antibody reactivity is against the core protein of HCV (C 22-3).
Serum HCV RNA
HCV RNA is detected in serum by the polymerase chain reaction (PCR), usually with primers derived from the more conserved 5′ untranslated region of the viral genome. Although theoretically this is the more direct and sensitive way to establish the diagnosis of ongoing
HCV infection, the technique is expensive and difficult to standardize, with unsatisfactory specificity and reproducibility in many laboratories. These problems have been solved only in part following the development of diagnostic kits by the pharmaceutical industry. These assays still lack optimal specificity, reproducibility, and sensitivity. Serum HCV RNA testing is not superior to anti-HCV testing in establishing a diagnosis of hepatitis C in immunocompetent patients who have evidence of liver disease without other aetiological factors, anti-HCV remains positive over time while viraemia may fluctuate and occasionally falls below the detection limit (false negative). The clinical settings in which testing for serum HCV RNA is recommended for evaluation of hepatitis C. These include10.
- Individuals who are anti-HCV R1BA positive or indeterminate with normal ALT. Serum HCV RNA allows the distinction between current and past infection. Eighty to ninety per cent of RIBA positive patients are serum positive for HCV RNA. Most of these individuals have histological evidence of chronic hepatitis, despite the normality of liver enzymes. RIBA positive, HCV RNA negative subjects are interpreted as cases of past infection if they are confirmed negative for HCV RNA on repeated testing. Patients who are RIBA indeterminate and show isolate reactivity against C22 may have HCV RNA in their serum as evidence of ongoing infection. In contrast, cases with isolated reactivity against C100-3 or 5-1 epitope are almost invariably HCV RNA negative and the low level ELISA reactivity is most likely nonspecific.
- Patients in the early phase of acute hepatitis C, before anti-HCV seroconversion. All patients with acute hepatitis of unknown origin should be tested for serum HCV RNA to assess whether they have acute infection with HCV.
- Immunocompromised or immunosuppressed patients in whom hepatitis C may be suspected. These patients may not produce anti-HCV or may develop low antibody titres of doubtful interpretation. This group includes transplant recipients. In patients who have received a liver allogralt, the HCV RNA test is the only reliable method to identify de novo infection or recurrence of hepatitis C.
- Patients negative for anti-HCV with essential mixed cryoglobulinaemia, which is often associated with hepatitis C but may not exhibit anti-HCV in serum.
- Patients negative for anti-HCV with ‘cryptogenic’ chronic hepatitis. Some of these patients may have seronegative hepatitis C, identified only by testing serum or liver HCV RNA.
- Patients positive for anti-HCV with autoimmune disorders, autoimmune reactions, or hypergammaglobulinaemia. The anti-HCV reaction may not be specific or may not be associated with ongoing infection. In patients with autoimmune markers and chronic hepatitis (chronic hepatitis positive for antinuclear or anti-LKM antibodies), testing for serum HCV RNA allows us to define whether liver damage is associated with HCV replication or is mainly due to an autoimmune reaction.
- Patients positive for anti-HCV with liver disease who have evidence of other aetiological cofactors such as alcohol abuse or metabolic disorders. Testing for serum HCV RNA allows us to define whether HCV contributes to liver damage.
- Neonates born to mothers who are anti-HCV positive. Anti-HCV is always found as a result of passively transmitted maternal antibodies. Serum HCV RNA testing represents the only way to establish whether transmission of HCV has occurred.
- Patients with hepatitis C treated with interferons or antivirals. Serum HCV RNA is useful to monitor the response to therapy.
Quantification of serum HCV RNA
Serum levels of HCV RNA can be measured to a number of laboratory procedures. Home made assays include endpoint detection competitive PCR and coamplification of synthetically mutated target RNA. These approaches are expensive, difficult to standardize, and often lack reproducibility. Commercial assays have also been developed. They are very expensive and not fully standardized; their use in the clinical setting is contoversial. They do not appear to give essential information in the diagnostic and prognostic evaluation of acute and chronic hepatitis C; a correlation between levels of viraemia and the acitivity or outcome of liver disease has not been established, and viraemia may fluctuate over time in the same individual, with or without a parallel behaviour of serum ALT. Particularly high levels of HCV RNA are found in the early phase of acute hepatitis C but also in the endstage phase of cirrhosis. Immunosuppressed patients often have high viraemic titres. A 10 to 100-fold increase in HCV RNA is usually observed after liver transplantation in HCV-infected patients, as a consequence of immunosuppression. The response to interferon therapy in chronic hepatitis C is significantly affected by the level of HCV RNA in the pretreatment serum10,33.
Recommended diagnostic/ clinical use of qualitative (PCR-based) serum HCV RNA tests10.
Diagnostic/clinical settings | Interpretation |
| Low-risk individuals with normal ALT and a positive or indeterminate anti-HCV RIBA | HCV RNA positive: ongoing hepatitis C, HCV RNA negative: past hepatitis C (?) |
| Patients negative for anti-HCV with acute hepatitis of unknown aetiology | HCV RNA positive: early acute hepatitis C HCV RNA negative: no hepatitis C |
| Iminunocompromised, immunosuppressed, transplanted patients with hepatitis who are negative for anti-HCV. Anti-HCV negative essential cryoglobulinaemia. Chronic hepatitis of unknown origin. | HCV RNA positive: anti-HCV seronegative hepatitis C or HCV infection |
| Patients positive for anti-HCV with chronic liver disease and present of aetiological damage, cofactors (autoimmunity, alcohol abuse, metabolic disease etc.) | HCV RNA positive: is a cofactrs of liver damage |
| Neonates born to anti-HCV mothers | To assess transmission of HCV |
| Hepatitis C cases treated with interferons /antivirus | To monitor virological responses |
Clinical use of HCV markers and diagnostic algorithm
Based on the above information the following recommendations can be made for the use of HCV markers by the clinician. When acute hepatitis C is suspected, the detection of serum HCV RNA by PCR may have been diagnostic relevance during the early phase; anti-HCV seroconversion by second or third generation anti-HCV ELISA assays should be sought in later phase serum samples. IgM anti-HCV tests give inconsistent results and do not provide additional diagnostic or prognostic information10,13. The outcome after acute hepatitis C, can be assessed by sequential HCV RNA testing, and persistence of a positive PCR for more than 6 months after clinical onset is highly suspicious of a chronic evolution; a progressive reduction of anti-HCV titres in patients in whom ALT levels have returned to normal and cleared HCV RNA is indicative of full recovery from the infection.
In patients with a sustained ALT elevation and clinical and/or histological evidence of chronic hepatitis, anti-HCV by ELISA is sufficient to make the diagnosis of hepatitis C; supplemental anti-HCV or HCV RNA testing may not be required.
Identification of hepatitis C virus antigen by immuno-histochemical staining
In an effort to detect hepatitis C virus antigen in liver tissue the authors investigated the immunoreactivity to monoclonal antibodies on frozen liver tissue from a chimpanzee and patients with chronic non-A, non-B hepatitis. Monoclonal antibodies were developed in mice immunized with a synthetic peptide derived from hepatitis C virus core antigen. One monoclonal antibody was reactivated and showed typical cytoplasmic granules in chimpanzee hepatocytes. Using this monoclonal antibody a similar staining pattern was found in the liver biopsies of 21 out of 28 chronic non-A, non-B hepatitis patients, positive for hepatitis C virus RNA and anti-HCV34.
Groups for consideration of HCV testing6
- Persons with signs and symptoms of hepatitis.
- Patients with chronic hepatitis, cirrhosis or hepatocellular carcinoma.
- Persons with unexplained increase in serum ALT or AST.
- Intravenous drug abusers, or those with a history of past drug abuse.
- Transfusion recipients, patients with malignancies or thalassaemia.
- Recipients of blood products; haemophilia, Von Willebrand’s disease, other coagulopathies.
- Hypogammaglobulinaemics.
- Plasmapheresed patients.
- Sexually promiscuous people and prostitutes.
- Occupational exposure: Medical, dental and laboratory health care workers, those with needle stick injuries or those routinely exposed to blood products.
- Spouse or sexual partner of HCV infected person.
- Haemodialysis patients.
- Liver, bone marrow or kidney transplant recipients.
- Inmates of custodial institutions.
- Infants of anti-HCV positive mothers.
Diagnosis of Acute Hepatitis C Acute infection
The average incubation period of acute hepatitis C, as determined in prospective studies of post transfusion hepatitis, is 7 to 8 weeks, but the range varies widely (2 to 26 weeks). Shorter incubation periods were frequent in experimental infections and in individuals with haemophilia treated with factor VIII; in these patients incubation was described as short as 2 days. In prospectively followed transfusion recipients, viraemia was the first marker to become detectable during acute hepatitis C, it was demonstrated by sensitive PCR as early as I week after exposure. Viraemia then persists without an antibody response for a period (window phase) of a few weeks to several months; during this early period, serum HCV RNA titres are higher than those found during the chronic phase.
Using second or third-generation ELISA assays, anti-HCV seroconversion usually can be detected 4 to 8 weeks after exposure, but may be delayed up to several months, with large individual variations. Titres of serum HCV RNA decrease as anti-HCV becomes detectable. The various antiviral antibodies usually do not appear simultaneousy, but rather in sequence, most commonly, anticore is raised first, followed by anti NS3 and anti-NS4 (anti-ClOO). Other patterns can be seen and not all patients develop the full battery of anti-HCV reactivities.
The onset of liver damage, marked by variable ALT elevations, is always delayed with respect to the appearance of viraemia and may either precede or follow seroconversion to anti-HCV.
Patients rarely have prodromic symptoms or fever; some show a mild and brief elevation of ALT. The acute phase of hepatitis C is often mild or inapparent and usually less severe than hepatitis A and B; more than two-thirds of the cases are asymptomatic and anicteric. Only 25 per cent of prospectively followed patients with transfusion associated hepatitis C were icteric and less than 10 per cent were severely ill, while symptoms and jaundice have been more frequently reported in community acquired disease; the difference is probably not real, since in published studies most patients with the latter form were recognized because of clinical symptoms while the former group was identified prospectively during the follow up of blood recipients. A severe or fulminant course of acute hepatitis C occurs rarely, except in patients with immunodeficiency, with pre-existing liver disease, or with other cofactors such as hepatitis A or B or drugs. At present the role of HCV in causing fulminant hepatic failure remains controversial; there may be significant geographical differences, as HCV RNA was detected rarely in fulminant hepatitis cases in Western countries but frequently in cases in Japan.
The clinical symptoms of acute hepatitis C are those of any type of viral hepatitis and are often indistinguishable from hepatitis A and B. Symptomatic cases present with malaise, dark urine, nausea (with or without vomiting), abdominal discomfort, and/or jaundice. Hepatitis C exhibits several patterns of aminotransferase elevation; the most typical is polyphasic, with significant fluctuation of enzyme levels over time. Sometimes, phases of biochemical abnormality are separated by periods of ALT normality. Other patterns of ALT with prognostic implications are: (i) a monophasic pattern, with ALT persistently elevated without significant fluctuations, the acute phase often merging into a chronic outcome. Chronic hepatitis develops frequently in patients with the polyphasic ALT pattern.
Acute hepatitis C displays gammaglutamyl transferase levels some what higher than the other forms of viral hepatitis10‘35. The IgG fraction of the serum immunoglobulin is often increased without significant changes in the IgM fraction; the latter is more affected than IgG in other forms of acute viral hepatitis. The histological features of acute hepatitis C include a few peculiar morphological changes, in addition to the classic features common to viral hepatitis in general. Liver biopsies may show eosinophilic clumping of the cytoplasm, macrovescicular steatosis, marked activation of sinusoidal cells, piling up of bile ductular cells in the lumen, and large numbers of acidophilic bodies. These changes coexist with a lymphocytic infiltration that is less prominent than in hepatitis A and B. Despite characteristic findings, a liver biopsy is not recommended for routine diagnosis of acute hepatitis C as none of the histological lesions is specific. A liver biopsy is not essential to identify progression to chronicity; this can be diagnosed by monitoring ALT levels and serum HCV RNA in the months after acute disease79. By definition, patients with abnormal ALT values for more than 6 to 12 months have progressed to chronic hepatitis C and may at this point undergo a liver biopsy to establish the histological activity of the disease. Also, patients who remain serum positive for HCV RNA despite ALT levels returning to normal, often have evidence of chronic hepatitis, usually of mild activity, on liver biopsy. A subacute course of hepatitis C with progressive hepatic failure is exceptional and should always stimulate a search for other causes of liver damage.
The most frequent pattern of evolution is that of a progressive reduction of ALT levels after the early acute phase, often with transient prolonged periods of normal levels followed by relapses of enzyme activity. Serum HCV RNA is persistently or intermittently positive. Sequential evaluation of the patient for at least 6 to 12 months is essential to define the outcome of acute hepatitis C correctly.
Pattern A
In this type there is full recovery with virus eradication. This favourable outcome is thought to occur in 10 to 30 per cent of infected individuals. The percentage with self-limited infection has varied in different series, as a consequence of the type of patients included and of the criteria used to define recovery. The persistence of a normal level of ALT after the acute phase is not an acceptable criteria for recovery as chronic infection may develop with normal ALT. Furthermore, a negative HCV RNA test in a single serum sample may not guarantee virus eradication, because of the possibility of intermittent viraemia during chronic infection. Some studies have gone to the extreme of denying that full recovery of acute hepatitis C with complete virus eradication is possible, others have described such cases. Patients with the recovery become negative for HCV RNA and may loose all anti-HCV reactivities over the long term. The RIBA patients may become indeterminate with an isolated reactivity to C22 better anti-HCV becomes negative. In some studies, recovery was more frequent in patients with community acquired hepatitis C no overt parenteral exposure compared with post-transfusion hepatitis, but this was not continuation in other studies. Resolution of acute hepatitis C may also depend on the viraemic title and genomic heterogeneity of the quasispecies in the infecting inoculation and on the HCV genotype.
Pattern B
In this type there is persistent viraemia with normal ALT levels. In these patients ALT remains normal after acute hepatitis while the HCV RNA remains continuously or intermittently positive with transition into a chronic HCV carrier state. About 10 to 20 per cent of patients with acute hepatitis C develop this profile. They usually maintain high anti-HCV titres over the long term. The natural course of chronic infection in these cases is only partially known and will be described under chronic infection.
Pattern C
In this type there is a progression to biochemically active chronic hepatitis. This is the most frequent pattern, with 40 to 60 per cent of patients with acute hepatitis C progressing to chronic hepatitis C, biochemical activity is shown by the ALT behaviour10,35. The enzyme profile may remain continuously abnormal or exhibit ALT peaks and elevations separated by phases of a normal level that last from months to
a few years. Conceivably, the asymptomatic HCV carrier state and biochemically active chronic hepatitis C may represent different phases of the same disease rather than two separate clinical entities. Viraemia may be persistent or intermittent, with large fluctuations in litres often, but not always, mimicking the behaviour of ALT. The long-term outcome in these patients is described under chronic infection.
Post-transfusion hepatitis C
Serologic testing now indicates that seroconversion to anti-HCV occurs in 85-100% of patients with chronic post-transfusion NANB hepatitis. In studies of post transfusion hepatitis in Spain, seroconversion to anti-C 100-3 occurred 6-8 weeks after transfusion in 38%, 20-26 weeks after transfusion in 56% and 38-52 weeks after transfusion in 6%. In some patients a very early appearance of anti-HCV may be due to possibly acquired antibody from the donor blood.
Anti-HCV persists for years and even decades in chronic hepatitis C, but may decline in titre or disappear with resolution. During the early phase of primary HCV infection, serum HCV RNA is the only diagnostic marker of infection and RNA testing therefore remains the only means of diagnosis in seronegative patients. Unfortunately, the test is only available in research laboratories. Serum HCV RNA has been detected within 1-3 weeks of transfusion in patients with hepatitis C, and usually lasts less than 4 months in patients with acute self-limiting hepatitis C but may persist for decades in patient with chronic disease.
Histological features: Acute hepatitis C
The histopathology of acute hepatitis C differs little from that of infections caused by the other hepatitis viruses and liver biopsy is not a usual investigations in acute viral hepatitis unless the diagnosis is in question. The histological features of acute hepatitis C are similar to those seen in hepatitis A or B, except for more marked sinusoidal cell activation. The distinction from evolving chronic hepatitis C can be difficult. The major pathological features are those of acute hepatitis. Liver cell swelling, acidophil body formation, cholestasis and infiltration of sinusoids by lymphocytes are the main abnormalities but the appearance-vary according to the timing of the biopsy, the severity and clinical outcome.
The inflammatory infiltrate is composed of lymphocytes, macrophages and plasma cells. Pale cell (ballooning degeneration) are noted. Cells with acidophilic cytoplasm (acidophilic cells) are seen and are an often-important hallmark of acute hepatitis.
Subacute hepatic necrosis or fulminant hepatitis is characterized by extensive hepatic necrosis. The organ may shrink as a result of extensive necrosis. The different causes of acute hepatitis are difficult to separate morphologically. In acute hepatitis A, periportal inflammation and necrosis and perivenular cholestasis are prominent. In acute hepatitis B, HBsAg can rarely be found histochemically. Staining for HBcAg is usually associated with active viral replication and is more commonly seen in chronic hepatitis B. Prediction of chronicity of a case of hepatitis C can’t usually be made from the biopsy appearance. In early infection striking lymphocytic portal reaction occurs, and the extensive lobular changes of chronic infection make the distinction between acute and chronic infection quite difficult.
Diagnosis Of Chronic Hepatitis C
Usually chronic HCV injection is not preceded by an overt acute episode. Two different biochemical profiles are recognized: (i) the HCV carrier state with persistent (or intermittent) viraemia and normal ALT, (ii) chronic hepatitis C with ALT abnormalities. The majority of cases will have been preceded by an episode of clinically apparent, icteric hepatitis and most hepatitis C carriers are unaware of their disease and their potential infectivity. Between 50 and 75% of patients with type C post-transfusion hepatitis continue to have abnormal serum aminotransferase levels after 12 months and chronic hepatitis histologically. The risk of chronic infection after sporadic hepatitis C is probably similar.
Most patients with chronic hepatitis C are asymptomatic or only mildly symptomatic. In symptomatic patients, fatigue is the most common complaint, and is variously described as lack of energy, increased need for sleep or fatiguability. Many patients do not give a history of acute hepatitis or jaundice. Physical findings are generally mild and variable and there may be no abnormalities. With more severe disease, spider angiomata and hepatosplenomegaly may be found. Serum aminotransferase decline from peak values encountered in the acute phase of the disease, but typically remain increased by two to eight folds.
The serum ALT concentrations may fluctuate over time and may even intermittently be normal. Many patients have a sustained elevation of serum aminotransferase. The relationship between HCV RNA in serum and serum aminotransferase is complex and although most patients with raised serum ALT are HCV RNA positive the converse is not always true. Indeed, probably half the patients with chronic HCV infection have normal or only minimally elevated serum aminotransferase concentration. Nevertheless these patients can have a marked degree of hepatitis as indicated by histologic evidence despite normal serum aminotransferase concentration6,35.
The spectrum of chronic disease varies. Most patients appear to have indolent, only slowly progressive course with little increase in mortality after 20 years. However, cirrhosis develops in approximately 20% of patients with chronic disease within 10 years, although the cirrhosis may remain indolent and. only slowly progressive for a prolonged period. The disease is not necessarily benign however, and rapidly progressive cirrhosis can occur. Older age at infection, concomitant alcohol abuse and concurrent HBV or HIV infection or other illness may be important aggravating co-factors. With the development of cirrhosis, weakness, wasting, oedema and ascites become more common. Older patients may present with complications of cirrhosis, or even HCC. With progressive disease, the laboratory values become progressively more abnormal. The finding of aspartate aminotransferase (AST) greater than ALT, low albumin and prolongation of PT suggest cirrhosis. Low levels of auto-antibodies may also become detectable.
Serological diagnosis: Chronic hepatitis C
Anti-HCV antibodies persist in the majority of patients with chronic post-transfusion NANB hepatitis, with serological testing showing a high prevalence of anti-HCV in patients with chronic active hepatitis and/or cirrhosis considered to be due to NANB hepatitis. The majority (75-95%) of patients with post-transfusion chronic NANB hepatitis in the USA and Europe are positive for anti-HCV. The disease in many patients with chronic NANB hepatitis may or may not be associated with a history of blood transfusion. The prevalence varies according to the background endemicity of hepatitis C in the population. Tests for anti-HCV are now important in establishing a diagnosis of what formerly considered cryptogenic cirrhosis.
In studies in Chimpanzees, anti-HCV (i.e. anti-C 100-3) was not neutralizing, in that primates with high levels of this antibody were also shown to have high titres of circulating hepatitis C virus. The development and maintenance of current diagnostic antibodies to hepatitis C virus therefore appears to reflect concomitant virus replication and consequently a high potential for infectivity.
A proportion of patients may improve spontaneously, but the number of patients who do so is unclear. These patients lose antibody after follow-up of at least 5 years and usually develop normal serum aminotransferases. Other patients may show a decline in anti-HCV titre with time. HCV RNA usually persists in patients with abnormal serum aminotransferase and anti-HCV. However, HCV RNA, and hence viraemia can also be found in patients with normal liver function tests. Isolates of HCV in individual patients may show nucleotide substitutions with times suggesting that the HCV RNA mutates at a rate similar to other RNA viruses. Preliminary but unconfirmed reports have suggested the HCV antigens can be detected in liver biopsy preparation in chronic carriers.
Histological features
The histopathology of chronic hepatitis C has more characteristic histologic findings. Three main pathological forms of chronic hepatitis such as chronic active hepatitis, chronic persistent hepatitis and chronic lobular hepatitis, occurs in hepatitis C6,35. These refer to certain defining morphological features that may have prognostic implications. However, these diagnosis are not separate entities and do not take into account the dynamic process of chronic hepatitis over time. Nonetheless, these definitions are in widespread use. Typically patients with chronic hepatitis C have relatively mild chronic hepatitis histologically. Lymphoid follicles in the portal tracts, bile duct damage, lobular activity including acidophiic cells and lymphocytes in sinusoids and fatty change are the most common features.
The single most characteristic feature is the lymphoid follicle, ranging from loose aggregates of lymphocytes to well defined structures with germinal centers. Although follicles are not restricted to hepatitis C, they are often pointers that alert the pathologist of the possibility of HCV infection.
Mild chronic hepatitis C may persist for many years without evidence of progression to severe chronic hepatitis. The patient’s health remains good. In more severe chronic hepatitis C, several patterns of necrosis are seen, including spotty focal necrosis or confluent and bridging necrosis. This may link either portal veins or the terminal hepatic venules. Hepatic venules linking portal tracts, known as central portal bridging, may represent more severe lesions with a strong likelihood of developing cirrhosis. In patients with long standing chronic hepatitis, fibrotic change and progression, to cirrhosis may occur6,35.
It is not easy to project the prognosis for patients seen at one point in time. Episodes of hepatic necrosis may progress at variable rates to cirrhosis and conversely the lesion may revert in some patients to inactive hepatitis. Cirrhosis develops after chronic hepatitis rather than following massive hepatic necrosis and may develop in patients with mild histologic pattern. The mechanism for the transition is not known but it may occur after repeated attacks of lobular necrosis associated with piecemeal necrosis.
However, a relationship between histological exacerbations and episodic clinical course is not proven.
The morphological features of cirrhosis due to hepatitis C are not specific to the disease; in the early stages, lymphoid aggregates may be seen. Interestingly, routine screening of blood donors for anti-HCV indicates that a significant proportion of asymptomatic anti-HCV positive blood donors indeed have progressive liver disease. Several histological studies in asymptomatic anti-HCV positive donors have shown that 45-62% have chronic active hepatitis and 7-15% have active cirrhosis. Progression to HCC is also well documented and despite the indolent and slowly progressive nature of the disease in many, it is apparent from serological testing for anti-HCV that HCV is a leading cause of morbidity from liver disease in the western world.
HCV And Other Liver Diseases
Hepatitis B and HIV
HCV may cause disease concurrently with hepatitis B, particularly in risk groups such as hemophiliacs and drug abusers, or where these diseases are endemic in the same environment6‘36‘74. The combination may cause aggravated disease. Likewise, in drug abusers and hemophiliacs, HIV and HCV may coexist and cause severe, accelerated liver disease.
Autoimmune hepatitis
There are conflicting reports regarding the occurrence of hepatitis C antibodies in patients with autoimmune liver disease. Clearly ELISAs for anti-HCV are prone to false positive results in patients with high concentrations of immunoglobulins in serum. These false reactive anti-HCV antibodies in patients with anti-smooth muscle antibody may actually disappear with immunosuppressive treatment as globulin levels decrease. However, Italian patients with autoimmune chronic active hepatitis appear to have a high frequency of genuine exposure to HCV, whereas seropositivity in English patients usually represents a false positive result. Therefore, it is uncertain whether anti-HCV in patients with chronic autoimmune hepatitis represents persistent anti-HCV from earlier disease, whether autoantibodies in autoimmune hepatitis patients cross react with HCV related antigens.
Up to 50% of patients with type II autoimmune hepatitis (anti-liver kidney microsomal antibody positive) are anti-HCV positive, and anti-HCV and anti-LKM in association may also represent another example of molecular mimicry. Anti-HCV positive patients with anti-LKM autoimmune chronic active hepatitis are usually male, are older, and have lower liters of anti-LKM than patients without anti-HCV. The target antigen of antibodies to LKM is a portion of the cytochrome P450 II D6 molecule, anti-LKM is not directed to a c 100-3 epitope, but some sequence homology between HCV and cytochrome P450 may exist. Autoantibodies, frequently in low titer, are also found in 15% to 20% of patients with chronic hepatitis C infection. These associations have some therapeutic implications, as the autoimmune disease is responsive to corticosteroids and may be aggravated by IFN. It is preferable to confirm HCV viraemia in these patients by RT-PCR.
In Japan, 80% of patients with chronic hepatitis C have circulating antibodies to a pentadecapeptide (Gor), an epitope of normal hepatocytes. This phenomenon may represent an autoimmune response peculiar to HCV.
Alcoholic Liver Disease
In several countries, a higher prevalence of anti-HCV has been found in patients with alcoholic liver disease. The prevalence of hepatitis C infection in this group correlates with the severity of liver injury and is higher in patients with cirrhosis than in those with only fatty change. The relationship is complex, which apparently reflects in part the higher rate of blood transfusions in alcoholics with decompensated liver disease and a common environmental risk.
HCC
Serological analysis of patients with HCC has shown a high prevalence of anti-HCV in patients with HCC. Several case-control studies have suggested that up to 78% of male patients with HCC are anti-HCV positive. The highest prevalence of HCV in HCC is found in Italian, Japanese, and Spanish patients; the prevalence is lower in Chinese and African patients, in whom the disease is more commonly associated with chronic hepatitis B. Because HCV is an RNA virus, it is believed that chronic HCV infection induces necroinflammatory change that progresses to cirrhosis and eventual malignant transformation, rather than an insertional oncogenic event. In Japan, at least, a history of transfusion has been documented in 42% of anti-HCV positive patients with HCC. The mean interval between the date of transfusion and the diagnosis of cirrhosis and HCC is usually long, 21 and 29 years, respectively. The role of concurrent HBV and HCV infection in development of HCC in cirrhotic patients has been studied and found that concurrent HBV and HCV infection predisposes cirrhotic patients to a significantly higher risk of HCC than patients with single agent infection37.
GBV-C or Hepatitis G Virus
Recently a new virus, termed GB virtis-C or hepatitis G virus, has been identified and may be responsible for some cases of non-A-E hepatitis. The prevalence of this virus in patients with chronic HCV is relatively high, at up to 25%, which probably reflects common risk factors. The clinical significance of co-infection is unclear37.
Management
Antiviral therapy is recommended for acute and chronic HCV infections3,38. The ultimate goal of treatment is to achieve the sustained eradication of the virus. However, this objective is difficult to attain and other parameters such as normalization of serum ALT, reduction of HCV RNA to undetectable levels by PCR and restoration to normal histology of the liver have been considered. In the majority of cases the total eradication of the virus is not achieved. However, therapy may reduce the risk of acute cases becoming chronic and suppress the disease progression from chronic active hepatitis to cirrhosis, reducing the risk for hepatocellular carcinoma. Long term antiviral therapy may also minimize the chances of relapse and exacerbation of histologicu! activity. Treatment may reduce extrahepatic manifestations. There is, therefore, a strong argument to treat chronic active hepatitis (CAH) cases. There are reports that therapy may not only prevent but can reverse fibrosis38.
Even before HCV was identified as the chief etiologic agent in non-A, non-B hepatitis, interferon alfa therapy was associated with normalization of alanine aminotransferase levels in some persons who were given this diagnosis38. In 1989, the first cases of successful treatment of documented HCV infection with interferon alfa were reported, but the very high rates of relapse frequently necessitated retreatment, which was almost invariably unsuccessful. A number of different interferons have been used, but all appear to have similar efficacy. Although the introduction of combination therapy with interferon and ribavirin has markedly improved clinical outcomes, less than half of those with HCV infection can be expected to have a favourable response to the agents that are currently available39.
The success of these therapies can be measured in terms of a biochemical response (normalization of alanine aminotransferase levels), but the introduction of new assays for the detection of viral RNA now allows the assessment of a virologic response (as defined by a negative result on a qualitative PCR assay for HCV RNA). Some clinical trials have also assessed the histologic response, but in clinical practice there is little indication for post-treatment biopsy39.
Since responses to therapy may not be maintained after treatment is stopped, the success of clinical trials has been evaluated in terms of the response at the end of therapy (end of treatment response) and six months after the cessation of treatment (sustained treatment response). Persons with a sustained virologic response have a high probability of having a durable biochemical, virologic, and histologic response.
Treatment of acute viral hepatitis
Although hospitalization may be required for clinically severe illness, most patients do not require hospital care. Forced and prolonged bed rest is not essential for full recovery, but many patients will feel better with restricted physical activity. A high calorie diet is desirable and because patients may experience nausea late in the day, the major calorie intake is best tolerated in the morning. Intravenous feeding is necessary in the acute stage if the patient has persistent vomiting and can’t maintain oral intake. Drugs capable of producing adverse reactions such as cholestasis and drugs metabolized by the liver should be avoided. If severe pruritus is present, the use of the bile salt sequestering resin cholestyramine will usually alleviate this symptoms. Glucocorticoid therapy has no value in acute viral hepatitis C. In fact this therapy may be hazardous.
Physical isolation of patients with hepatitis to a single room and bath room is rarely necessary except in case of voluminous bleeding for hepatitis C. Burdensome enteric precaution are unnecessary but emphasis should be placed on blood precautions i.e. avoiding direct, ungloved hand contact with blood and other body fluids. The importance of simple hygienic precautions, such as hand washing, can’t be overemphasized. Universal precautions that have been adopted for all patients apply to patients with viral hepatitis C8,39.
Data regarding the efficacy of the treatment of acute HCV infection are very limited, since the infection is seldom diagnosed during the acute phase. Given the high rate of progression to chronic infection and the relatively limited efficacy of therapy for chronic infection, the treatment of acute infection has been advocated, but it has not yet prove to be beneficial. Furthermore, some patients with acute symptomatic HCV infection have high rates of spontaneous clearance and would therefore be treated unnecessarily39. However the preliminary results of more recent studies suggest, that early treatment, even with interferon alone, has a high rate of efficacy. In view of these data early therapy may be advisable, but the optimal therapeutic regimen and the best point at which to intervene have not been defined. Since the study of persons with acute HCV infection may also provide valuable information about the pathogenesis of HCV infection in general, it would be ideal to follow such patients in controlled clinical trials.
Another unanswered question is whether postexposure prophylaxis for example, after a needle stick injury is beneficial, as is the case for HIV-1 infection. Currently, no prophylactic regimen has been shown to be effective and efficient, and only monitoring is recommended.
In view of the high rate of pregnancies of HCV infection treatment of such hepatitis C has been advocated in order to prevent chronicity. Some very good results have been reported. Ten of 1 1 patients with acute hepatitis C treated with a mean total of 52 no of interferon B. In 30 days maintained normal ALT after 3 years compared to only 3 of 14 untreated patients, responding cleared HCV RNA.
Although there is no evidence that treating patients during the primary phase of HCV infection is more beneficial than treating them after the conventional criteria of chronicity have been acquired, their is also no evidence of a diminished or detrimental effect of interferon in patients with acute hepatitis C. Therapy should be instituted as soon as hepatitis C recognised10,39.
Treatment of chronic hepatitis C
Current therapy for well compensated chronic hepatitis C consists of antiviral agents and immunomodulatory agents aimed at altering viral replication and modify the immune response of the host6,8,40.
Initial management of patients with chronic hepatitis C consists largely of counselling, particularly regarding the natural history of the disease and methods of avoiding transmission. Frequently the patients experience marked anxiety once the condition is diagnosed and this may be related to exaggerated reporting in the mass media and to previous contradictory advice from health care professionals and others.
Indications and recommendations for antiviral therapy for chronic hepatitis C8
| Standard indications for therapy |
| Elevated ALT activity Fibrosis or moderate to severe hepatitis on liver biopsy Delectable HCV RNA |
| Retrenchment Recommended |
| Relapse after an initial course of interferon A 6 month course of combination interferon-ribavirin or a course of interferon monotherapy longer in duration than the original course |
| Antiviral therapy not recommended routinely but decisions made on individual basis |
| Children (age <18 years) Age >60 Mild hepatitis on liver biopsy Compensated cirrhosis Patients with HIV infection and normal CD4 counts Maintenance therapy in repeated relapsers Non-responders to a course of recombinant interferon can be offered a 48 wk course of consensus interferon, 15 micro gram three times a week (clinical trials underway to assess benefit of long term “suppressive” therapy with other regimens) |
| Longterm therapy recommended |
| Cutaneous vasculitis and glomerulonephritis with chronic hepatitis C associated |
| Antiviral therapy not recommended |
| Decompensated cirrhosis Normal ALT activity |
| Therapeutic regimens |
| First-line treatment: INF-alpha 3 million units subcutaneously three times a week plus ribavirin 1000 mg/d (weight <75 kg) to 1200 mg/d (weight >75 kg) orally, Duration of therapy Genotype 1:48 weeks Genotypes 2 and 3: 24 weeks. |
| Alternative regimen: INF-alpha monotherapy 3 million units (of recombinant interferon alfa-2a/ or alfa 2b or 9 micro gram of consensus interferon) subcutaneously three times a week for 48 weeks (primarily for patients in whom combination therapy is contraindicated or not tolerated) |
| Features associated with reduced rcsponsiveness |
| Advanced histologic lesion (e.g. advanced cirrhosis) Long-duration disease Genotype I High level HCV RNA (>2 million copies/mL) High HCV quasispecies diversity |
Classification of Intcrferons (IFNs)
Since their discovery in 1957, both natural and recombinant alpha IFNs have been shown to have bioactivity that interferes directly or indirectly with viral reproduction at both biochemical and cellular levels. They have been shown to have immunomodulatory and antiproliferative properties as well. IFN can improve hepatic inflammation, reduce viral replication and normalize ALTs in HCV patients3,40.
Interferons are classified broadly as alpha (derived from monocytes and transformed B-lymphocytes), beta (produced from fibroblasts) and gamma (produced by activated T-helper lymphocytes). Although several forms have been isolated, only four, namely IFN-alpha 2a, IFN-alpha 2b, consensus IFN and lymphoblastoid IFN, are commercially available-and approved for the treatment of viral hepatitis. The IFN-alpha 2 gene has been cloned and inserted into bacteria. This has led to the production of recombinant IFN-alpha 2, which is marketed as Roferon (IFN-alpha 2a) and Intron (IFN-alpha 2b). The two recombinant IFNs differ by only one aminoacid residue3,40.
In the past, IFN has been used in the treatment of chronic HBV with a nearly 40% response rate but lately, Lamivudine has been preferred. In cases of chronic HCV infections, IFN monotherapy results in only a 10%-20% sustained response rate. Although about 40% of patients achieve response at the end of treatment, patients relapse in almost 50% of cases. Viral genotypes Ib and 4 infections seem to be poorly responsive to IFN treatment compared to genotypes 1 a, 2 and 3.
Ribavirin (1-beta-D- ribofuranosyl- IH-1, 2, 4-Triazole- 3-carboxamide), a synthetic broad spectrum guanosine like nucleoside, has been found to have inhibitory actions in vitro against a wide range of DNA and RNA viruses. Its mode of action is thought to include the inhibition of inosine 5′ monophosphate dehydrogenase, inhibition of viral replication by interfering with RNA polymerase modulation of immune response by alteration of the levels of Th-1 and Th-2 cells and direct cytoprotection leading to decrease of hepatic inflammation.
The drug has been used in an aerosol formulation to treat influenzae A and B viruses and respiratory syncytial virus infections. Ribavirin marketed as Rebetol in the treatment of chronic HCV infection has been attempted but with a rather poor response rate. In certain cases beneficial effect on biochemical marker such as serum aminotransferases and improvement of liver histology such as hepatocellular necrosis and inflammation but not fibrosis have been reported. However, ribavirin monotherapy is not associated with a significant reduction in HCV RNA levels, bringing into doubt if the drug has any antiviral effect. The reported beneficial effects are not sustained after the end of therapy and all hepatitis C patients treated with ribavirin monotherapy relapse. It is therefore, the synergistic effect of the ribavirin and IFN that provides the advantages of combination therapy, and IFN monotherapy is being superseded by combination therapy with ribavirin in the treatment of chronic hepatitis C.
Mechanism of action of IFN
Interferons are a family of intracellular proteins that have established antiviral and immunomodulatory properties. Alpha interferons are natural glycoproteins produced in the cells in response to infection by viruses including HCV12,40. Interferons have many biologic-effects that might explain their activity in this disease. They bind to specific cell surface receptor and inhibit the replication of a wide spectrum of RNA and DNA viruses including hepatitis viruses through several mechanisms, including inhibition of virus attachment and uncoating, induction of intracellular proteins (gene) and ribonuclease and other enzymes that give the cell antiviral properties and affects viral replication, assembly and cell entry. Interferons also increase natural killer cell activity, enhance maturation of cytoloxic T cells and increase cell surface expression of HLA class I antigens. Thereby promoting immune clearance of infected cells. Interferon acts in hepatitis C predominantly through poorly understood direct antiviral effects. Its immunomodulatory actions, important in hepatitis B are less significant in hepatitis C. Its direct antiviral effect is evidenced by prompt drop in HCV RNA and ALT seen in responders, without the characteristic ALT flare seen in hepatitis B. Interferon has been shown to be effective in normalizing liver tests, improving hepatic inilammation and reducing the amount of viral replication in these patients.
Trials of Alpha interferon
Pilot studies of alpha interferon were begun before the identification of HCV, but nevertheless demonstrated that therapy was followed by marked improvements in serum aminotransferase in upto 70% of patients6,41. Subsequently multiple randomized controlled trials have documented that a 6 month course of alpha interferon in doses of 2-5 million units (MU) thrice weekly leads to remission in disease in approximately 50% of patients.
Results of prolonged treatment with interferon-alpha in chronic hepatitis C10.
Author | Interferon(Mu) | Dosage | Duration(month) | Primary response % | Sustained response % | Relapse rate % |
| Chemello | rec 2a | 6-3 | 12 | 72 | 36 | 49 |
3 | 12 | 55 | 40 | 31 | ||
6 | 6 | 74 | 62 | 28 | ||
3/ weekly | ||||||
| Rcichard | rec 2b | 3 | 15 | 60 | 37 | 37 |
| Poynard | rec 2b | 3 | 18 | 45 | 22 | 50 |
3-1 | 18 | 27 | 10 | 63 | ||
3 | 6 | 30 | 8 | 73 | ||
| Kasahara | Natural | 5 | 6 | 62 | 33 | 46 |
5 | 12 | 63 | 53 | 14 | ||
| Lin | rec 2b | 3 | 6 | 61 | 20 | 69 |
5 | 6 | 64 | 17 | 73 | ||
3 | 24 | 55 | 59 | 46 | ||
These remissions were marked by a fall in serum aminotransferase into the normal range and improvement in liver histology, Particularly the degree of hepatocellular necrosis and inflammation. Retrospective analysis demonstrated that this decrease in aminotransferase activities was accompanied by a fall in HCV RNA levels in blood and that most patients who developed normal aminotransferase on therapy become HCV RNA negative. Examination of stored serum samples with virologic assays showed that therapy with IFN alpha usually led to rapid decrease in HCV RNA levels and some patients had evidence of long term eradication of virus. Unfortunately, most studies of alpha interferon in chronic hepatitis C found a very high relapse rate once therapy was stopped. The relapse was accompanied by a return of serum HCV RNA and progressive liver injury. Thus, in most studies, the rate of long term responses to alpha interferon has been only 10-25%. Preliminary results from two European trials provide conflicting result on whether higher doses and more prolonged courses of interferon result in appreciably higher long term response rates6,41.
Initial evaluation
Therapy should not be started until a firm diagnosis of chronic hepatitis C is made. Chronic hepatitis C can be diagnosed on the basis of finding persistent elevations of aminotransferases, anti-HCV6,41 in serum and liver histology compatible with chronic hepatitis. Liver Biopsy should be performed before treatment (Except in haemophiliacs) to determine the baseline activity and to exclude subclinical cirrhosis and other causes of liver disease. Alanine aminotransferase level per se are not useful in determining need for treatments as they correlate poorly with histology and unlike hepatitis B, don’t predict response to treatment. Nonetheless pretreatment elevation of ALT is required to fulfil current guidelines. In addition the presence of elevated ALTs prior to treatment provides a ready means of monitoring response. Patient should have firm serological or epidemiological evidence of hepatitis C. Current assays for anti-HCV are some what problematic.
A small percentage (5-10%) of patients with chronic HCV infection will test negative for anti-HCV, even by second generation enzyme immunoassays (false negative results). Furthermore, a proportion of patients with other forms of liver diseases will test positive for anti-HCV (false positive). With future improvements in assays for anti-HCV these problems will lessen. At present some form of confirmatory test is needed to verify the diagnosis. Thus, the presence of a compatible history or epidemiological features (exposure to blood or blood products), the finding of HCV RNA in serum or HCV antigen in liver or the application of confirmatory assays for anti-HCV such as western blot or neutralization are valuable in confirming the diagnosis. A pregnancy test, thyroid function tests, auto antibodies (antinuclear antibodies, smooth muscle antibodies and thyroid auto-antibodies) and full blood count (FBC) should also be performed prior to therapy6,41.
Criteria for Interferon treatment14
- Age greater than 18 years.
- Histologically proven chronic hepatitis (not crirrohsis). Patients with inherited coagulation disorder do not require liver biopsy.
- Positive anti-HCV tested twice over six months with an interval of at least 16 weeks.
- ALT greater than 1.5 times the upper limit of normal, for more than six months.
- Alcohol usage of no more than 7 standard drinks per week.
- No illicit use of injectable drugs within previous 12 months. May be on methadone programme if dosage stable or reducing during previous 12 months.
- Not pregnant or likely to become pregnant (unless adequate contraception).
- Absence of HIV infection.
- No history of autoimmune liver disease.
- No history of significant psychiatric history.
- Treatment must cease if ALT remains greater than upper limit of normal after 12 weeks of therapy at 3 million units three times weekly.
Initial treatment regimens
Monotherapy for HCV infection with interferon alfa was associated with initial rates of response as high as 40 percent, but the rates of sustained response are less than half this41. This is especially true in persons infected with HCV genotype la or 1 b, the most prevalent genotypes in the Unites States and western Europe. Two large, prospective trials demonstrated that the combination of interferon alia and ribavirin significantly increase the percentage of previously untreated patients who have a sustained virologic response, from 16 percent to 40 percent. Both studies showed that in patients infected with HCV genotype 2 or 3 and in those with low viral loads before treatment, the response was maximal after 24 weeks of treatment, whereas patients infected with genotype 1 and those with a high viral load before treatment required a course of 48 weeks for an optimal outcome. This finding led to the recommendation that the duration of treatment should be based on the HCV genotype and the pretreatment viral load. However, since tests for the quantification of HCV RNA are still not standardized, and since the viral load naturally fluctuates over time, the viral load is currently not routinely used for determining the treatment regimen.
The treatment of persons with chronic HCV infection is based largely on consensus guidelines. The 1999 recommendations suggest that previously untreated persons with the above described indications and without contraindications to treatment with interferon or ribavirin should receive combination therapy. Treatment consists of 3 million U of interferon alfa administered subcutaneously three times a week and 1200 mg of ribavirin orally per day for patients who weight at least 75 kg and 1000 mg of ribavirin orally per day for those weighing less than 75 kg. Usually, ribavirin is taken in divided doses, given in the morning and evening, and interferon is given before bedtime.
The virologic response to combination therapy should be assessed at week 24, since elimination of the virus can occur late with this approach. Persons with a positive PCR assay for HCV RNA at week 24 should be considered to have had no response to treatment, and therapy should be discontinued. Those infected with HCV genotype 2 or 3 who have a negative PCR assay for HCV RNA can also usually stop therapy at this time, but an additional 24 weeks of treatment is suggested for patients with other genotypes and a negative PCR assay.
Contraindications to treatment with interferons alfa and ribavirin.
Contraindication | Intcrferon alfa | Ribavirin |
| Absolute | Current psychosis or a history of psychosis. Severe depression Neutropenia or thrombocytopenia. Symptomatic heart disease Decompensated cirrhosis Uncontrolled seizures Organ transplantation (other than liver) | Pregnancy Absence of use of a reliable form of contraception. End stage renal failure Anemia Hemoglobinopathies Severe heat disease |
| Relative | Autoimmune disorders (e.g. thyroiditis) Uncontrolled diabetes | Uncontrolled hypertension Old age |
Dosage and side effects
Ideally, IFN either as a monotherapy or in combination with ribavirin must lead to a low host toxicity and high viral clearance rate. Many studies have assessed the impact of different treatment schedules, responses and side effects. Three main regimens, namely, longer duration therapy, higher fixed doses and intensive dose induction, have been applied. Patients with favorable predictive factors of response, such as short duration of the disease, viral genotypes 2 and 3, low viral load, absence of fibrosis and cirrhosis, may respond to a standard combination regimen for six months, but this must not be considered as a rule since other factors such as age and sex may play an important role. On the other hand, those with high viral load, genotypes Ib and 4, longer duration of disease, presence of fibrosis and compensated cirrhosis may require higher dose regimens and longer treatment duration of 48 weeks to reduce relapse and obtain improved sustained response. Generally, high doses are associated with more adverse reactions and patients must be well monitored3‘41. Induction dosing, in which one large dose or more frequent doses are applied at the beginning of treatment, has been attempted, especially among IFN non-responders and relapsers, but it is still too early to predict its effectiveness.
A variety of side effects are associated with IFN monotherapy. Influenza-like symptoms such as fever, headache, fatigue, arthralgias and myalgias predominate in nearly 60% of patients at the early stage of treatment. These symptoms are well tolerated in many patients and can be ameliorated by acetaminophen. Severe adverse events such as depression, suicidal ideation, suicide and hypothyroidia, though uncommon, should not be ignored and may require dose modification or discontinuation of therapy. Other adverse events such as pharyngitis, insomnia, dyspnea, pruritus and nausea are less frequent and may be managed by dose reduction. Side effects associated with PEG-IFN are usually mild to moderate, similar to those of IFN monotherapy and manageable with dose adjustment. The symptoms appear to decrease in severity as treatment continues3,41.
Ribavirin is associated with major side effects, such as anemia due to extracellular hemolysis and suppression of bone marrow release of erythroid elements. The anemia usually reaches its nadir within the first four weeks of treatment. Anemic HCV patients must therefore be excluded from ribavirin treatment. Regular monitoring of hematological indices is warranted, especially during the first 4 weeks of treatment. Lymphopenia, gastrointestinal complaints and CNS defects have also been implicated. If hemoglobin concentration falls to lower than 10 g/dl, dosage must be reduced by 50%. Reduction of hemoglobin to below 8 g/dl should lead to cessation of treatment. The drug is teratogenic and strict attention to birth control is warranted in women of childbeariim age throughout the treatment period and six months after.
Side effects of treatment with interferon alfa and ribavirin.
Frequency of side effect | Interferon alfa | Ribavirin |
| 30% (every common) | Influenza like symptoms Headache Fatigue Fever Rigors Myalgia Thrombocytopenia Induction of autoantibodies | Hemolysis Nausea |
| 1-30% (common) | Anorexia Erythema at injection site Insomnia Alopecia Lack of motivation Inability to concentrate Irritability Emotional liability Depression DiarrheaInduction of autoimmune disease Leukocytopenia Taste perversion | Anemia Nasal congestion Pruritus |
| <%(rare) | Polyneuropathy Paranoia or suicidal ideation Diabetes mellitus Retinopathy Optic neuritis Hearing impairment Seizures Loss of libido Cardiotoxicity | Goul |
Follow up
While receiving interferon patients should be questioned about side effects and followed up 1 to 4 weekly with clinical assessment, ALT and FBC6,41. The frequency of visits should be based upon the severity of the liver disease and the degree of side effects experienced. PCR for HCV RNA is usually performed at the commencement and completion of treatment. Follow up should be at least 6 months after stopping therapy to assess whether the response obtained is sustained. Follow up liver biopsies are not needed unless retreatment is considered.
In hepatitis C, serum aminotransferase generally decrease during interferon therapy. Indeed, a worsening of serum aminotransferase due to worsening of hepatitis (probably due to induction of autoimmune injury to the liver) is a reason for early discontinuation of therapy. Several cases of acute exacerbation of hepatitis C with alpha-IFN therapy have been reported and some of these have severe and even life threatening. The cause of exacerbation is unclear; some patients were found to have acute immune hepatitis rather than hepatitis C as initially suspected.
If the values of aminotransferase are not normal after 3 months, a beneficial response is unlikely and therapy should be discontinued. Monitoring the level of HCV RNA may be more accurate means of assessing the response to therapy, if viral RNA is still detectable after 2 to 3 months of therapy, a long term response is unlikely and therapy should be stopped.
Level of transminase are measured after two months of treatment with interferon (3 million units three times a week) to determine the course of action.
Treatment of Chronic Hepatitis C Virus Infection with Interferons:
For the foreseeable future and until specific inhibitors of HCV replication are introduced, IFNa will remain the primary treatment modality for chronic HCV, either alone or together with the antiviral ribavirin (as combination therapy)41. After 12 to 18 months of standard dose IFN therapy, 15 to 20% of patients with chronic HCV achieve a long-term or sustained virologic response, as defined in the following. Evidence suggests that patients who respond can expect prolonged clearance of HCV RNA from the serum as well as a lowered risk of developing cirrhosis and hepatocellular carcinoma. Nevertheless, IFN has important drawbacks in terms of cost (in the United States more than $700 a month) and side effects. Furthermore, more than two thirds of the patients treated with IFNa do not achieve a sustained response, and most have relapses after IFNa is stopped. About 15% may never respond despite repeated courses of IFNa. However, studies have confirmed the safety and improved efficacy of combination therapy (IFNa plus ribavirin) over IFN monotherapy in patients who have had relapses. The new “CONSENSUS” interferon may be superior to combination therapy in efficacy, for specific subgroups such as those with cirrhosis of genotype Ib or both. Clinical trials are in porgress to compare the two forms of therapy. It remains to be seen whether daily doses of IFNa for the first month or two (so-called induction therapy) with or without ribavirin will provide additional benefit as initial therapy or for treating relapsers of nonresponders or both41.
Standard and Novel Types of Interferons Used for Treating Hepatitis C
In the United States, three a-interferons have been approved by the FDA for treating chronic HCV infection: IFN-a2b, IFN-a2a, and interferon alfacon-1, also known as CONSENSUS INTERFERON (CIFN, Infergen, Amgen, Thousand Oaks, Calif)41– IFN-a2b and IFN-a2a are human recombinant interferons produced by using a strain of Escherichia coli bearing genetically engineered plasmids that contain an IFN-a2b or IFN-a2a gene derived from human leukocytes. In contrast, CIFN is a synthetic interferon developed by selecting the most common amino acid at each position from 11 naturally occurring IFN subtypes with the most activity against HCV. CIFN has been reported to have 10-fold greater in vitro activity against HCV than IFN-a2b or IFN -a2a. It is also important to note that the dose of CIFN is measured in macrograms rather than million units. In general, a 9 m gm dose of CIFN is corresponding to 3 MU of IFN-a2b. These a interferons are administered subcutaneously three times a week and each has its own FDA-approved indications41.
Another new IFN-a is interferon alfa-nl, which is derived from a human B lymphoid cell line after induction by the Sendai virus. This unique IFN-a consists of over 22 components, 18 of which are similar to other IFN-a subtypes, and at least two additional proteins that are glycosylated, in contrast to IFN-a2b and IFN-a2a, which are single unglycosylated proteins. The optimal dosing schedule appears to be 3 MU subcutaneously three times a week for 12 months, which may actually be more effective than standard-dose IFN-a2b or IFN-a2a. Higher doses of IFN-a nl may lead to more adverse effects.
Pegylated interferon alpha
Hoffman-La Roche (Switzerland) and Schering Plough (USA) have recently developed a new generation of IFN by covalently linking a branched methoxy polyethylene glycol moiety to IFN. These new drugs (Pegasys and Peg interon) have been found to have a decreased systemic clearance rate, improving the serum half-life by ten-fold without altering the properties of the parent compound. Their biological activity, as measured using serum 2, 5- oligoadenylate synthetase activity, is prolonged.
As a result, PEG-IFN can be administered only once a week, which may reduce many of the initial side effects as well as minimize the surges in serum HCV RNA levels seen with thrice weekly dosing. One type of PEG-IFN, pegylated 40k IFN-a2a has shown promise in a phase II trial in which 155 noncirrhotic HCV positive patients were randomized to receive PEGASYS in doses of 45, 90, 180, or 270 mg. In patients with chronic hepatitis C, a regimen of pegiinterferon alfa-2a given once weekly is more effective than a regimen of interferon alfa-2a given three times weekly.
The outcomes of recent phase II trials showed a 76% virologic response at the end of a 12-week treatment and a sustained response rate of nearly 40% with Pegasys. Again, the response seems to be genotype dependent, since 28% of those infected with genotype-1 and 56% of genotype 2 and 3 attained sustained response. Clinical studies with Pegintron have reported a 25% virological sustained response. Pharmacodynamic and pharmacokinetic profiles of the new drug are improved compared to IFN, and preliminary studies have shown that PEG-IFN injected once a week is more efficacious than IFN injected thrice weekly. The new pegylated interferons are into phase III clinical trials and are expected to be available commercially in the near future. The above preliminary results suggest that the combination of PEG-IFN with ribavirin may be more effective than IFN plus ribavirin. Clinical trials using PEG-IFN and ribavirin have been started worldwide3,41.
One important study from 1999 is this European meta-analysis of six controlled trials of combination therapy in hepatitis C Interferon and ribavirin in combination were compared with interferon monotherapy for chronic hepatitis C. Response to therapy in these studies was defined as normal alanine aminotransferase level and viral clearance at the end of 6 months of completion of therapy and 6 months after therapy.
This meta-analysis collected and standardized the advantages of combination therapy for chronic hepatitis C. It also demonstrates that cirrhotic patients can tolerate combination therapy as well as patients without cirrhosis can and it shows a small but measurable benefit from combination therapy even in the presence of fibrosis.
Several years ago, it was noted that interferon treatment leads to improvement in fibrosis and histologic findings, even in patients who do not achieve biochemical or virologic response. Because ribavirin is teratogenic and cannot be given for long periods, longer term monotherapy with interferon is now being considered to retard the progression of fibrosis and reduce the risk for hepatocellular cancer in patients with chronic hepatitis C.
Amantadine:
The addition of amantadine to the regimen of IFN and ribavirin have shown better response than INF plus ribavirin therapy1.
A large National Institutes of Health trial is testing the strategy of long term interferon monotherapy in the prevention of progressive liver disease and hepatocellular carcinoma in HCV infected patients. The approach could be made more tolerable by the use of the new pegylated interferon. This drug is given only once a week. In the next 2 to 5 years, interferon monotherapy in the form of pegylated interferon may be used as a chemopreventive agent for cirrhosis and cancer in many patients who previously had no biochemical or virologic response to interferon.
To treat or not to treat
The question of whether to treat or not to treat depends on many factors3,41. The decision should be made not only on clinical grounds but social and economic as well. Not all HCV infection leads to disease. The disease is characteristically slowly progressive, taking years if not decades to manifest. Large scale prospective natural history data comparing treated and untreated patients are lacking. The present consensus is to treat not the infection but the disease. Patients with symptoms whose liver biopsies confirm features of moderate to severe degree of necroinflammatory activity and fibrosis or those in the early phase of compensated liver cirrhosis must be treated. Healthy HCV carriers with negative HCV RNA, minimal grade of necro-inflammation or minimal fibrosis must be monitored but not treated. However, healthy
HCV carriers with persistent HCV RNA, but low histological activity index may benefit from the delay of the speed of progression to fibrosis if treated.
Patients whose cirrhosis is more advanced and/or decompensated or who have hepatocellular carcinoma or end-stage liver disease do not seem to benefit from treatment and must be considered for liver transplantation if they are found to be suitable.
It is imperative that all treatment is carefully monitored biochemically, histologically and virologically throughout the therapy phase. Pretreatment quantitative HCV RNA baseline must be established. A second sample taken 24 weeks post treatment must be analyzed for quantitative HCV RNA. In the patients showing no significant reduction of the HCV RNA, it may be necessary to change the treatment dosage, try pegylated IFN, or drop them from the treatment scheme3,41.
HCV genotype and percentage of sustained response10.
Genotype | IFN monotherapy (%) | IFN + ribavirin (%) |
| la | 15-20 | ? |
| Ib | 4-8 | 20 |
| 4 | 17 | 17 |
| 2 | 30-40 | 40 |
| 3a | 35-50 | 75 |
Treatment Response
Long term or sustained response may be defined as one in which the serum aminotransferase concentrations are normal and no HCV RNA is detectable for at least six months after the cessation of IFN therapy. By this definition the rate of sustained, long term response to standard regimen for IFN is only 10-20%6.
Pattern of response
Responses to alpha interferon therapy in chronic hepatitis C can be grouped into four patterns. Approximately 25% of all patients have a sustained beneficial response. In these patients, aminotransferase begin to fall within the first month of treatment and are often normal by 2-3 months. Simultaneous testing for HCV RNA in serum reveal that the fall in aminotransferases is accompanied by a loss of this marker of viraemia. Aminotransferase remain normal and HCV RNA undetectable even after therapy is stopped. Later relapses can occur but are not common and have been reported only in patients who had HCV RNA in serum despite normal aminotransferases6,10.
Another 25% of patients with chronic hepatitis C have a complete response while on alpha interferon but promptly relapse when the medication is stopped. These patients may become negative for HCV RNA on treatment, but redevelop this viral marker when interferon is stopped and aminotransferase rise. Disease activity eventually returns to the original level in these individuals, but some amelioration of disease has been reported in a percentage of these patients.
Retreatment is often considered in these patients, but should only be undertaken if alpha interferon therapy was well tolerated and if an altered regimen is given, using either a higher dose or a more prolonged course.
A third group consists of about 25% of patients with chronic hepatitis C who have a partial or transient response only. In some patients, serum aminotransferases decrease but do not become normal. In others serum aminotransferases become normal only transiently and then rise despite continuation of treatment. Such patients may manifest a fall of aminotransferase into the normal range if the dose of interferon is increased. Unfortunately, not all patients will be able to tolerate the increase in side effects that usually accompanies the higher dose. Most of these patients do not have a sustained amelioration of disease activity with treatment.
A final 25% of patients with chronic hepatitis C have no response to alpha interferon therapy at all. In these patients serum aminotransferase activities remain elevated and serum HCV RNA is present during treatment. The cause of the patient’s resistance to interferon effects is unclear. Treatment should be stopped early-after 2-3 months in these patients and they should not be given higher doses or repeated course.
Prediction good response to interferon treatment of chronic hepatitis
Unfortunately there are no reliable features that predict which patients is likely to respond to treatment, nor are there reliable features that can predict when a sustained response has occurred before therapy is stopped42. Only about 40% of infected patients appear to respond to interferon and the treatment is costly, parenterally administered and frequently associated with side effects. Thus efforts have been made to identify those patients with the greatest likelihood of responding to therapy so that others may be spared the inconvenience of a treatment with little chance of benefit. More appropriate selection of candidates could substantially reduce the cost of therapy. In addition to facilitating more appropriate selection of patients, accurate predictors of response could allow better comparison of treatment response in different treatment trials and might identify a group of patients in whom other therapeutic agents should be tested instead42. There are 2 varieties of predictors. Firstly, univariate statistics identify potential association but are unreliable prediction of outcome. Thus univariate associations, although statistically significantly, may not truly influence outcome. Secondly, it follows that predictors and risk factors are more appropriately identified by multivariate and risk factors are more appropriately identified by multivariate models. Most important factor that affected the efficacy of IFN is the amount of HCV and/or HCV genotype. A patient with HCV genotype Ib frequently showed a poor response to IFN treatment.
Factors associated with complete response to interferon therapy5,12,42.
Method | Favourable factors |
A. Univariate | |
| 1. Drug administration Dose 3 MU tiw Normal weight or high dose/m2 2. Demographics: Female sex Younger age (<45 yrs) History of IV drug use Sporadic Short duration (<5yrs) 3. Histology: Chronic persistent hepatitis Absence of cirrhosis or only minimal histologic evidence of fibrosis. Low concentrations of iron in liver tissue before treatment. 4. Laboratory Absence of Iron overload. Lower bilirubin 5. Virolgic Genotype Low scrum HCV RNA level | |
| 6. Negative for HIV infection 7. Mutation in the NS5, region of the viral genome. | |
| B. Multivariate | 1. Drug administration: Body wt <86 kg 2. Histology: Chronic persistent hepatitis. Absence of cirrhosis 3. Laboratory Lower serum ferritin Normal gamma-GT Low serum bile salts 4. Virologic: Genotype 2 or 3 (as opposed to genotype I) and low levels of genetic diversity of HCV (so called quasispccics) Low serum HCV RNA level (<l05/ml) 5-1-1 antibody on RIBA |
In large trials the two most important independent predictors identified have been the serum HCV RNA level and viral genotype.
Serum hyaluronan (hyaluronic acid) as a marker of liver fibrosis
Serum hyaluronan level correlated with the extent of liver fibrosis both before and after a interferon therapy, but not with the histopathological indices of liver inflammation or necrosis. Parallel changes in serum hyaluronan and liver fibrosis occurred; serum hyaluronan levels fell significantly in patients in whom fibrosis improved, increased significantly in patients in whom fibrosis worsened and did not change significantly in patients in whom fibrosis was unmodified. Furthermore fibrosis improved only when antiviral effect of a interferon was reflected by persistent normalization of serum ALT, although there was no correlation between serum hyaluronan levels and aminotransferase activities. Thus serum hyaluronan appears to be a non-invasive index of liver fibrosis43.
Liver fibrosis in hepatitis C
In HCV infection, age, alcohol consumption and male sex are more strongly associated with fibrosis progression than are virologic factors. Data point to the conclusion that patients with chronic hepatitis C should abstain from alcohol consumption. Antiviral therapy given early in the course of chronic hepatitis C of appropriate patients is also a consideration44. Study report suggests that increasing body mass index has a role in the pathogenesis of steatosis in chronic hepatitis C and that steatosis may contribute fibrosis. The association between body mass index and statosis and fibrosis has important prognostic and therapeutic implications in the management of patients with chronic hepatitis C virus45.
Interferon antibodies
Patients treated with alpha-2a interferon for chronic hepatitis C may produce anti-interferon antibodies whose effect, if any, on the individual response to therapy has not been fully clarified46. The prevalence and kinetics of anti-interferon, including those of neutralizating type, have been studied in 60 patients with chronic hepatitis C enrolled in a randomized controlled trial of recombinant alpha-2a interferon. Thirty patients received interferon while 30 were untreated controls. Two different methods, an enzyme immunoassay and an antiviral neutralization bioassay were used and serial serum samples from each patient were analyzed. Enzyme immunoassay positive anti-interferon appeared in 60.7% of treated patients within 6 months of therapy; antiviral neutralization bioassay positive anti-interferon appeared in 52.9% of these enzyme immunoassay positive patients, and was associated with high enzyme immunoassay reactivity and long term persistence. Anti-interferon was detected in 75%..of patients showing no response to interferon. Antibodies were also detected in 3 out of 6 patients who showed alanine aminotransferase normalization persisting upto the end of treatment and in 8 out of 14 patients who showed an initial marked reduction or even normalization of alanine aminotransferase, followed by reactivation of liver damage during treatment. Interestingly, patients who became anti-interferon positive before complete alanine aminotransferase normalization later showed reactivation of liver damage independently of interferon after complete alanine aminotransferase normalization either did not reactivate or did so only after interferon dose reduction46.
Approaches to Increasing the response rate
Most attempts to increase the response rate in chronic hepatitis C have focused on using higher doses (e.g. 5 MU or 10 MU) of IFN or more prolonged periods (e.g. 9, 12, 18 or 24 months) of treatment and or higher frequencies (daily doses) of therapies. In several European studies, duration of therapy of 12 to 18 or 24 months increased the frequency of sustained responses from 15% to more than 25% but this effect has not been observed as conclusively in studies conducted in the USA. The longer courses are .more expensive and cause more discomfort than 6 months course and relapse still occur40.
Patients with atypical forms of hepatits C
The current recommendations for alpha interferon therapy is for patients with typical well compensated chronic hepatitis C. The criteria for selecting patients eliminates some patients who may deserve treatment. Such as patients with asymptomatic chronic hepatitis C, acute hepatitis C, those with advanced cirrhosis, those with normal serum aminotransferase levels patients who are anti-HCV negative and those who are immunosuppressed6,46.
Treatment of asymptomatic patient with chronic hepatitis C
Although consensus exists that patients with symptomatic chronic hepatitis C should be treated with interferon, treatment of a symptomatic patients remains controversial6. Because progression to cirrhosis can occur in an unpredictable proportion of such cases the potential benefit of therapy should not be dismissed out of hand in these patients.
Additional study is needed. Currently, the treatment of asymptomatic hepatitis C “Carriers” with normal aminotransferase levels or patients with decompensated cirrhosis secondary to chronic hepatitis C is not recommended.
Acute hepatitis C
Acute hepatitis C represents approximately 25% of sporadic hepatitis in USA and western countries. The disease can be severe and prolonged, although it is rarely fulminant. Most importantly, however, acute hepatitis C commonly progresses to chronic hepatitis C with persistence of the viraemia and persistence of the hepatocellular injury. For these reasons, therapies of acute hepatitis C have focused on prevention of chronic disease rather than amelioration of the acute disease. Indeed, it seems logical that early intervention in acute hepatitis C is warranted to prevent chronicity and the injury of chronic hepatitis6,41.
In view of the high rate of progression of HCV infection treatment of acute hepatitis C has been advocated in order to present chronicity. Some very good results have been reported. Ten of 1 1 patients with acute hepatitis C treated with a mean total of 52 no of interferon B. In 30 days maintained normal ALT after 3 years compared to only 3 of 14 untreated patients, responding cleared HCV RNA.
Although there is no evidence that treating patients during the primary phase of HCV infection is more beneficial than treating them after the conventional criteria of chronicity have been acquired, their is also no evidence of a diminished or detrimental effect of interferon in patients with acute hepatitis C. Therapy should be instituted as soon as hepatitis C recognised.
Alpha interferon should not be used in routine case of acute hepatitis C. Therapy can, however, be considered in acute cases in which it becomes clear that the disease is not resolving normally. Thus, if serum aminotransferase remain elevated for more than 2 months after onset of illness, therapy is appropriate, especially since interferon is well tolerated during acute hepatitis C and can result in prompt improvement in serum aminotransferase. The optimal dose and duration of therapy is not known; it is reasonable to use the same regimen recommended for chronic hepatitis C:3 MU thrice weekly for 6 months.
Patients with decompensated liver disease
Among patients with hepatitis C who have cirrhosis the rate of sustained response following interferon therapy is only half that of patients without cirrhosis. Although it has been suggested that higher dose regime in patients with cirrhosis may improve response, this remains largely untested47. The results of a recent Australian study of cirrhotic patients who were given an intense interferon programme of 4.5 MU daily for 24 weeks were compared with previous studies of patients with hepatitis C. Of 11 studies of interferon response in chronic hepatitis C comparison of pretreatment variables showed considerable differences. Identification of predictors of response by univariate and multivariate analysis regularly indicated the importance of age and fibrosis. Analysis of six studies with either a poor (5% or less) or a reasonable (14-19%) sustained response rate to interferon in patients with cirrhosis suggested that a higher dose or longer duration of therapy was associated with better results. The experience of Australian study, where 14% of patients had sustained biochemical response to interferon and side-effects were reasonably tolerated with careful monitoring, suggests that future studies in cirrhosis should be carried out exploring higher doses and longer duration of therapy47.
Patients with normal aminotransferases
Patients with chronic HCV infection have various degree of liver injury. Many patients with this infection will have no elevations in serum aminotransferase. Some of these may have a degree of chronic hepatitis by liver biopsy. These patients are often detected when they have donated blood and been found to be anti-HCV positive. At present, one would not recommend treatment of patients with chronic hepatitis C who have normal or near normal serum aminotransferase activities. Chronic HCV infection can be benign and not associated with progressive liver injury. These patients should be followed and treated only if biochemical evidence of disease becomes manifest.
Children with chronic hepatitis C
There have been few reports of therapy of chronic hepatitis C in children. However, there is no reason to believe that children would be either more or less susceptible to IFN treatment and treatment is recommended in doses of 3 MU/m2 (up to 3 MU total dose) 3 times weekly for 6 months. The criteria’for treatment should be the same as those for adults. Most small children tolerates interferon therapy very well with no side effects except for moderate degrees of hair loss that is reversed within 3-6 months after therapy is completed6,47.
Atypical serological patterns
Some patients with chronic hepatitis C test negative for anti-HCV, and some of these may not have been clear cut evidence of exposure to hepatitis C. The diagnosis can be established by research assays such as those for anti-HCV by immunoblotting or for HCV RNA by reverse transcription polymerase chain reaction40. These patents can be treated, but only if there is clear cut evidence of HCV infection. There is need for caution because patients with autoimmune hepatitis who are mistakenly thought to have hepatitis C can exhibit a worsening of the hepatitis with interferon treatment. Autoimmune hepatitis is typically associated with the presence of hyperglobulinaemia and autoantibodies such as antinuclear antibody (ANA) and anti smooth muscle antibody (ASMA). However 10-20% of adults with chronic hepatitis C have ANA or ASMA or both reactivities and may, nevertheless, respond well to alpha interferon.
Chronic hepatitis C positive for serum markers of autoimmune disease
The serum autoantibodies, anti-nuclear antibody, anti-DNA antibody, anti-smooth muscle antibody, antithyroglobulin antibody, antimicrosomal antibody, antimitochondrial antibody, rheumatoid factor and antibody to deoxyribonucleoprotein were measured at the baseline and on completion of interferon a2a (IFN-a2a) treatment in chronic hepatitis C patients who did not present with any autoimmune disease prior to treatment of the 57 patients examined, 27 spontaneously manifested at least one autoantibody48. Only the prevalence of rheumatoid factor (20%) was significantly higher in the CHC patients than in the control subjects. There were no differences in the prevalences of the 8 autoantibodies examined between hepatitis C virus (HCV) genotypes, Ib and 2a/2b.
One problem connected with IFN therapy is induction of autoimmune disease. In this connection, it is not recommended to treat the patients with overt autoimmune disease with IFN, which may exacerbate them. However, how should we treat the patients positive for autoantibodies which are frequently associated with autoimmune diseases? The positivity for the antibodies alone does not affect the outcome of IFN treatment. It seems that the positivity for non-organ specific autoantibodies are of little value in the prediction of induction of autoimmune disease. When the autoantibody titer is very high, however, there is hesitation to use IFN. This is the case in some patients with CHC. Furthermore HCV infection induces chronic hepatitis which is difficult to differentiate from autoimmune hepatitis (AIH). This poses the important question of which to use as treatment IFN or corticosteroids (CS). IFN exacerbates AIH, whereas CS may increase HCV, though increase in HCV does not always aggravate hepatitis49.
Immunosuppressed patients
Patients with immune deficiencies or those receiving immunosuppressive medication are frequently infected with HCV and represent a difficult group of patients to manage6,49. Chronic hepatitis C is common among oncology patients, renal dialysis patients, patients with human immunodeficiency virus infection and patients who have received a solid organ transplant. Furthermore, the immunosuppression itself may worsen the underlying liver disease (Martin et al., 1989, 1991).
There have been no prospective, controlled trials of chronic hepatitis C in immunosuppressed patients. Small uncontrolled trials of therapy of patients who have both HCV and HIV infection suggest that interferon may ameliorate the chronic hepatitis. Furthermore, preliminary reports of therapy of patients with recurrent hepatitis C after liver transplantation suggest that alpha interferon can suppress viral replication and improve serum aminotransferases even in the face of large doses of immunosuppressive medications. The safety and relative benefit of interferon therapy in these situation is still unclear and warrants further study. At the present time, immunosuppressed patients should probably not receive therapy outside of prospective controlled trials.
Retreatment of interferon of chronic hepatitis C
Most of the respondences to a first course of interferon who relapse will respond to a second similar course, but only a minority achieve a permanent remission. A change in the type of IFN may after some benefit to patients exhibiting an ALT break through during the first course10,51.
The role of more aggrieve therapy is controversial. In the studies, higher doses of interferon more prolonged treatment did not produce any effort in non respondences. However in another study of 90 patients who did not respond in a sustained fashion to a first therapeutic course, 25 classified as non respondences did not develop a sustained response regardless of whether they received the same schedule on a high dose on a longer duration therapy, of the other 65 patients classified as relapses, 20% developed a sustained response upon treatment; of those who received of MU their weekly of 6 months and were retreated with 6 MU for 6 months followed by 3 MU for a further 6 months, 4% became sustained responders of the retreatment. In patients relapsing after a successful first course, retreatment appears more likely to induce a sustained response in cases with undetectable HCV RNA of the end of initial cycle10,51.
Role of Liver Transplantation
HCV cirrhosis is one of the leading indications for orthotropic liver transplantation in North America and Europe, and short term survival after transplantation is similar to that of patients with chronic cholestatic liver disease. Liver transplantation should be considered in patients with decompensated hepatitis C cirrhosis. Reinfection of the graft with hepatitis C is almost universal, most likely due to extrahepatic sites of HCV replication. Despite often very high HCV RNA levels post transplantation, the resultant liver disease is frequently only mild and early graft survival is equivalent to that for other indications of transplantation52,53,61.
Liver transplantation
Liver transplantation is the only available treatment option for patients with decompensated HCV related cirrhosis and is also indicated for some patients with early stages of hepatocellular carcinoma. Reinfection of the graft with HCV is nearly inevitable, and the majority of patients will have histologic signs of hepatitis and even cirrhosis54,61. Despite these drawbacks, the one-year and five-year rates of survival of HCV infected persons who undergo liver transplantation do not significantly differ from those of patients with other common indications for liver transplantation. New therapies are necessary to improve the long term outcome of liver transplantation, either to prevent infection of the liver transplant or to treat it effectively.
After liver transplantation for chronic hepatitis C, recurrence of viremia is almost inevitabfe. High viral loads, the frequent presence of type Ib infection, and immunosuppression considerably reduce the chance of a sustained antiviral response in this group. Maintenance therapy with antiviral therapy may be helpful, Interferon, despite the theoretical risk of graft rejection, appears well tolerated, and there are also anecdotal reports of improvement with short term ribavirin monotherapy or ribavirin/ interferon combination regimens. Although ribavirin as monotherapy is an attractive option for maintenance therapy in this group of patients, little is known about the long term toxicity.
Allograft rejection
Evaluation of the impact of rejection on patients with recurrent HCV have been limited, and interpretations are difficult because treatment of rejection requires intensified immunosuppression, which may directly alter the natural history. Retrospective and perspective studies (188, 189) have shown that about 40% of patients develop hepatitis with allograft damage, as early as 1 to 3 years after transplantation. This however rarely leads to graft loss or death within 5 to 10 years of transplantation.
The impact of recurrent hepatitis C on long-term patient survival is controversial. Adult survival data in the USA indicate that 5 year survival among patients transplanted for hepatitis C is inferior to that of patients transplanted for autoimmune hepatitis or cholestatic liver diseases (e.g. primary biliary cirrhosis and primary sclerosing cholangitis)53. However, it is comparable to the survival of patients with alcoholic liver disease and superior to the survival of patients transplanted for hepatitis B or HCC.
Prognosis
The prognosis in chronic HCV infection varies greatly and is difficult to determine. Probably 20 to 30% of patients ultimately have cirrhosis and disability from end stage liver disease8‘72. Hepatitis C occurring after transfusion is less severe during acute phase than type B hepatitis and is more likely to be anicteric; fatalities are rare but the precise case fatality rate is not known.
There is a definite but unquantitated risk of HCC. The response to alpha interferon therapy is 35 to 50% with a relapse rate of 20 to 25%. Controlled trials of IFN- a for chronic hepatitis C subsequently found that a six months course of therapy led to decrease in serum aminotransferase concentrations in the majority of patients and to normalization of the values in 40 to 50%, whereas the condition of untreated patients rarely improved. When therapy was stopped, however serum aminotransferase concentrations rose pretreatment values in at least 50% of patients who responded to the drug. So the rate of sustained response was only 15-25%8,14,72.
After acute HCV infection, the likelihood of remaining chronically infected approaches 85%. Although many patients with chronic hepatitis C have no symptoms, cirrhosis may develop in as many as 20% within 10 years of acute illness, in some series of cases, cirrhosis has been reported as many as 50% of patients with chronic hepatitis C.
Although the chronic hepatitis C accounts for a quarter of cases of chronic liver disease and a quarter of patients undergoing liver transplantation for end-stage liver disease in United States and Europe, in the majority of patients with chronic hepatitis C, morbidity and mortality are usually limited during the initial 20 years after the onset of infection. The risk of HCC is increased in patients with chronic hepatitis C, almost exclusively in patients with cirrhosis and almost always after at least a decade, usually after three decades of disease8,72.
Prevention
It is now clear that the hepatitis C virus HCV is widespread and eventually may be responsible for a significant proportion of viral liver disease in many populations. Before the introduction of the screening of blood donations, it probably accounted for between 75% and 90% of cases of post-transfusion NANBH (PT-NANBH). Furthermore, a high percentage of individuals in high-risk categories, 60% to 85% of hemophiliacs, 50% to 75% of intravenous drug users, and 10% to 20% of hemodialysis patients are, or have been, infected with the virus.
Transmission of HCV
The transmission of HCV may conveniently be considered in relation to the specific route of infection and the particular groups of individuals at risk of infection.
Although the mechanism of transmission of HCV is likely to be similar to that HBV, it is clear that there are some significant differences. Data suggest that in developed countries the sources of HCV infection, taking all infected individuals together, can be approximated as follows: in 20% to 40% of patients, the source of transmission is undefined; 35% to 45% of patients have a history of intravenous drug use (IVDU); 10% to 20% have been directly exposed to the virus; 5% to 15% of patients have a history of transfusion; and up to 5% are health care workers.
- Intravenous drug users
- Recipients o virally untested blood and blood derivatives
- Recipients of virally untested organ and tissue transplants
- Individuals having tattoos or piercing
- Those infected by undefined routes (spordic or community acquired with no readily identifiable risk factors)
- Close contacts, including occupational, of individuals infected by owe of the other routes
It is clear that transfusion of infected blood and blood products is a particularly efficient route of transmission of HCV and indeed of other blood-borne infectious agents, there is now clear evidence that before screening was introduced at least 80% to 90% of cases of PT-NANBH were due to HCV infection. Although there is a large amount of information concerning both the transmission of HCV through blood and blood products and the prevalence of HCV in blood donors, only 0.5% to 10% of unscreened blood (red cell or single-donor product transfusions have been found to result in identified PT-NANBH6,80.
Transmission to Patients Receiving Products Derived from Large Pools of Plasma
This group largely comprises hemopholia A and B patients, whose high risk of infection through infected blood products has long been recognized because of their high donor exposure. The effectiveness of virally inactivated factor VIII concentrates will be discussed later in the section concerned with the prevention of HCV infection; however, emerging data do indicate that effectively treated products do not transmit HCV infection. Between 60% and 100% of hemophiliacs who have at any time received untreated products are anti-HCV positive.
Look-back studies
After the introduction of blood screening, a number of look-back studies are, or have been, undertaken to identify and investigate patients transfused with blood or products donated by donors subsequently identified as HCV infected. Although important, such studies make some key assumptions that must be considered when analyzing the data generated, namely, that the product was actually transfused and to the patient indicated, donations before the index HCV positive donation were infectious, evidence of infection resulting from the transfusion of potentially infected material (can be resolved by genotyping if RNA is available from donor and patient). In an early look-back study, Kerner et al found that 60% of recipients of blood from donors subsequently found to be HCV positive had evidence of HCV infection, although they do not state the number of patients who may have already been infected with HCV.
Transmission by organ and tissue transplantation
There is now evidence to suggest that HCV infection may be transmitted through organ and tissue transplatation6. Post-transplantation liver disease is still an important cause of morbidity and mortality, especially in renal transplant recipients, in whom NANBH has been considered to be a significant cause. In an attempt to define the role of HCV infection in post-transplantation liver disease, Pereir et al. screened a group of 716 organ donors for anti-HCV.
Transmission by undefined routes
In up to 40% of cases of HCV infection, the precise route of infection cannot be defined. In infected individuals with no clearly defined risks, sporadic or community-acquired infection needs to be considered, raising the possibility of less obvious routes of infection. Generally, these could include sexual contact, maternofetal transmission, and close but nonintimate personal contact.
Sexual transmission
By analogy with HBV, sexual transmission (especially homosexual) would appear to be a likely mode of spread of HCV. Although a number of studies have addressed the question sexual transmission of HCV, results are somewhat conflicting. However, the weight of evidence does seem to favor some degree of sexual transmission, the presence of anti-HCV has been demonstrated in 11% to 35% of spouses or sexual partners of HCV infected patients, significantly higher than in other family members and the general population.
Mother-to-child transmission
Although, like a number of other aspects of HCV, the vertical transmission of HCV from mother to infant was suspected, most of the early studies did not confirm this route. More recent studies have now clearly shown that mother child transmission does occur and is one of the factors responsible for the maintenance of the occurrence of sporadic or community acquired cases of HCV infection, for example by subsequent horizontal infant-infant transmission.
It is not certain whether transmission to the infant occurs in utero by infection via the placenta during the development of the fetus, during birth by direct infection with virus present in the cervical secretions, or in the perinatal period through breast milk. All three routes are well documented for methods of mother to child infection of a number of infectious agents, and transmission of HCV may thus be possible by any one (or more) of these routes.
Transmission to nonsexual close contacts
Several cases of infection through nonsexual household contact have been reported. Kiyosawa et al. studied the family members of patients with chronic HCV liver disease and found that 15 of 195 (8%) of the family members of anti-HCV positive patients were anti-HCV positive. Bellobuono et al found that the prevalence of anti-HCV in family members of anti-HCV positive blood donors was 7.3% compared with 0.62% in the local blood donor population.
Transmission through nonhuman vectors
Transmission through nonhuman vectors, notably arthropods, is a well-defined route for some infectious agents. Yellow fever virus, the prototype flavivirus, is transmitted by this route. Currently, there are no confirmed reports of nonhuman transmission of HCV; however, Bellavita et al. have considered nonhuman vectors as a possible route of infection.
Occupational contact
Some individuals may be at increased risk of infection by a number of infectious agents by the nature of their occupation. In these cases, transmission is invariably by exposure of infectious body fluids following direct and close contact with infected individuals. Needlestick injuries in nursing and medical staff are probably the most common cause of occupational exposure in the clinical environment. A number of cases of needlestick transmission of HCV infection have been reported in nursing and medical staff.
Prevention Of HCV Infection
General preventive measures
Prevention of the transmission of any infectious agent requires knowledge of the routes of infection to identify the points at which preventive intervention would be most effective. The problems of prevention of transmission of HCV are similar to those of HIV and HBV, in which a number of different routes are involved and a combination of direct and indirect measures are required.
A vaccine to protect against HCV infection is not yet available and may take some time to develop. Passive protection using high titer specific immunoglobulin is also currently unavailable, although the production of such a preparation may be feasible. At present the role of specific HCV neutralizing antibodies in the prevention and control of infection has not been defined clearly. Difficulties in preparing a protective vaccine are the following: (a) only man and Chimpanzee are infected and better animal models are needed; (b) HCV replicates poorly in vitro (c) The viral envelope proteins (E1/E2) are highly mutable; antibodies against then fail to provide long team protective immunity56. Currently, therefore, prevention of HCV transmission can be achieved only by preventing exposure to the virus. This may either be by indirect means – screening blood and organ donors for evidence of infection- or by direct means – physical prevention of exposure to the virus.
Prevention of transmission by blood transfusion
The prevention of transmission of any infectious agent by transfusion of blood or blood products can be approached in a number of ways. All approaches are directed toward minimizing the exposure of transfusion recipients to the infectious agent, there are four ways of achieving this goal.
Self Exclusion
Ensuring the safety of donated blood begins with the identification of the safe donor. Safe donors are those who, by virtue of their normal behavioural patterns, are at low risk of parenteral or sexual infections. Potential blood donors are provided with sufficient information to be able to decide not to donate if they think they have been exposed to infectious agents as a result of fairly well-defined risk activities.
Serological screening of donated blood
Transfusion- transmitted infections are largely preventable if the appropriate screening programs are designed and implemented. As described previously, the use of the screening assays themselves should form only one part (albeit a significant part) of an overall strategy for ensuring the safety of transfused blood and its derivatives.
Primary anti-HCV screening
The effectiveness of blood donor screening to detect infectious agents depends on the sensitivity and specificity of the primary assays. The sensitivity and specificity of any assay are influenced by a number of factors, one of the important ones being the nature of the antigens used. Following the original work of Choo et al. further HCV proteins have been identified and proposed structure for the complete genome has been constructed. Most commercial screening assays are based on a combination of proteins: recombinants and peptides from one or more of the core, NS3, NS4, and NS5 regions6,80.
Detection of IgM Anti-HCV
A number of groups have studied the value of detection of IgM antibodies to HCV to try to resolve some of the above mentioned problems. Classically, the early appearance of IgM antibodies in viral infections has been used as an indication of probable acute infection, and where IgM appears earlier than attempt to close the window period between initial HCV infection and the detection of anti-HCV, a period during which only HCV RNA may be detectable.
Confirmatory (Supplementary) Assays
At present, confirmatory assays for HCV serology are not available commercially. Those that are currently available make use of essentially the same antigens used in the primary screening assays. Although they still have limitations, assays such as RIBA has proved very useful in interpreting the bulk of enzyme-linked imimmosorbent assay (ELISA) reactive samples6,80.
PCR
PCR has revolutionized diagnostic virology. Although its high sensitivity can also highlight problems of cross contamination, it provides the potential for the direct detection of the presence of the infectious agent in any tissue. Using reverse transcriptase (RT) PCR, the RNA genome of HCV can be detected in a wide range of sample types.
Inactivation of Infectious Agents in Blood products
Although blood transfusion services have adopted comprehensive measures to minimize transfusion- transmitted infection, these are not nor can they be, totally effective. Blood may be donated during the window period of a recent infection or markers of infection may be subliminal. Such events are rare, but the relative risk is magnified in products made from large pools of plasma if they are not virally inactivated.
Appropriate Use of Blood
As discussed earlier, even with an appropriate and effective screening program, each transfusion always carries a slight residual risk of transmission of any one of a number of infectious agents.
Prevention of Transmission by Organ Transplantation
In keeping with blood transfusion policy, organ donors should be screened for evidence of HCV infection before transplantation.
Prevention of Transmission by Intravenous Drug Users
HCV infection in intravenous drug users is transmitted parenterally through contaminated syringes and needles. HCV transmission can be minimized by educating intravenous drug user not to reuse or share syringes and needles or any other items involved in drug taking.
Prevention of Sexual Transmission
Transmission through sexual contact can be minimized by protected sex. Although the risks of sexual transmission have not yet been quantified and no specific marker of risk of sexual transmission is available, there is a clear defined risk.
Future Prospects
Given the present trends, HCV infection will continue to have a global impact on health in the foreseeable future. The high rates of progression to chronic infection and the lack of effective means of prevention require that HCV infection be differentiated from other causes of viral hepatitis. Despite recent progress, efforts to develop more effective therapies must remain a high priority. Worldwide, the best hope for a solution to the epidemic of HCV infection is the development of an effective vaccine. Although the recent demonstration of apparent immunologic clearance of virus in some persons with acute infection provides hope that a vaccine may some day be developed57,58,80. It is not likely to be available soon. For those who are already infected with HCV, new therapeutic approaches can be expected in the future. Persons who have no response to therapy and who have a high risk of imminent progression to decompensated liver disease might benefit form therapies that halt disease progression until better therapies become available, in a small study, interleukin-10 had beneficial effects on liver abnormalities59,80. This approach, as well as the long-term administration of low doses of interferon or ribavirin, is currently being evaluated in large, prospective studies. With the better characterization of the replicative cycle of HCV, it should be possible to develop virus-specific inhibitors that work in manner analogous to that of inhibotors of HIV-I replication. Potential targets include the HCV proeases helicase and polymerase as well as the internal ribosomal entry site or the putative cell-surface receptor CD81. In the meantime, HCV infection will undoubtedly remain a clinical challenge throughout the world.
Inngenetics (Belgium) has started a phase 1 Clinical Study using a therapeutic hepatitis C. vaccine capable of stimulating the immune system to produce antibodies that not only prevent primary infection but also eliminate the virus in HCV carriers.
Several agents, such as ursodeoxycholic acid, N-acetyleysteine, NSAIDS and others, have been proposed as adjuncts to IFN treatment, but none have shown to be of significant benefit. Several researchers are of the notion that multidrug approaches, as with HIV treatment experience, may be our best prospects. New drugs such as amantadine/rimantadine, protease/helicase/polymerase inhibitors and antisense onligonucleeotides are being, actively pursued and the design of specific inhibitors, similar to those used for HIV treatment, are in the offing. However, the virus is very heterogeneous with a high mutation rate drug resistance will be expected in the future. Tipple therapy of IFN, ribavirin and amantadine has been attempted on nonresponders, but the results are still awaited.
Conclusion
Chronic HCV infection is a major cause of chronic liver disease and HCC worldwide. The many sporadic cases that occur in patients with no identifiable risk factors, the propensity of the virus to cause subclinical chronic hepatic injury and the lack of definite therapy or prevention will probably result in many cases of advanced liver disease secondary to HCV infection well into future.
In conclusion, where as public measures may be successful ‘in preventing new infections, treatment will continue to be an option for old infections. For HCV chronic carriers without symptoms, regular monitoring of liver functions and periodic measurement of HCV-RNA should suffice. No treatment is necessary in this group. For chronic HCV carriers with symptoms such as chronic active hepatitis and compensated liver cirrhosis, combination therapy must be considered. However, the present treatment regimens are still experimental, with poor sustained responses, and are expensive and not without side effects. Patients contemplating treatment must be fully aware of the need for frequent hospital visits, strict compliance to drug refinements and long-term monitoring3,80.
Treatment without monitoring is fruitless and wasteful: Specialized referral centers with facilities for biochemical, virological and histological monitoring must supervise all treatment. Combination therapy of IFN and rivavirin is becoming the standard treatment. For non responders and relapsers, more potent antiviral drugs and their combinations are the likely direction in the future, The new pegylated interferons are being used in combination with ribavirin successfully. We are hopeful that future clinical trials in this region will be given the support they truly deserve. hepatitis C as a disease will continue to persist, and so will the challenge to combat the infection by combination therapy.
Further research into the virology, immunology, molecular biology and epodemiology of HCV and development of better cell culture systems and better animal models will be essential in enhancing our understanding of this virus and defining rational approaches to the treatment and prevention of HCV infection.