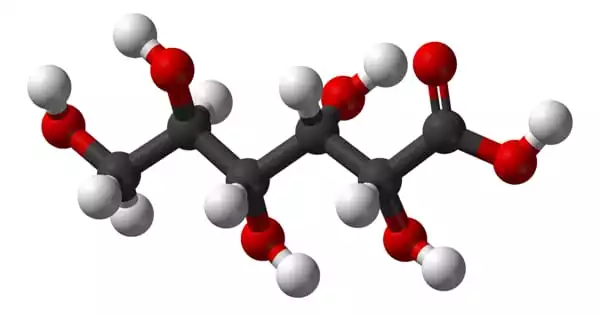
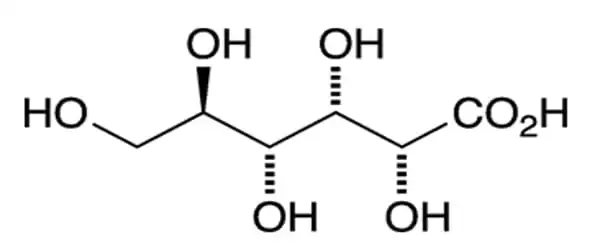
Gluconic acid has the molecular formula C6H12O7 and the condensed structural formula HOCH2(CHOH)4COOH. It is an antiseptic and chelating carboxylic acid formed by the oxidation of the first carbon of glucose. It is one of the sixteen stereoisomers of 2,3,4,5,6-pentahydroxyhexanoic acid. It has a brown clear appearance and is noncorrosive, nontoxic, and mild organic acid. It is highly soluble in water and has a mild, refreshing flavor.
At neutral pH, gluconic acid forms the gluconate ion in an aqueous solution. The cyclic ester glucono delta lactone structure in aqueous gluconic acid solution chelates metal ions and forms very stable complexes. The salts of gluconic acid are referred to as “gluconates.” This agent has strong chelating activities towards anions in alkaline solution, including calcium, iron, aluminum, copper, and other heavy metals. Gluconic acid, gluconate salts, and gluconate esters are abundant in nature because they are formed by the oxidation of glucose. Some drugs are administered as gluconates.
Gluconic acid is abundant in nature, particularly in fruits and sucrose-containing substances like honey. It is abundant in plants, honey, and wine and can be commercially prepared through a fungal fermentation process. This agent and its derivatives can be used as additives or buffer salts in the formulation of pharmaceuticals, cosmetics, and food products.
Chemical structure
The chemical structure of gluconic acid is a six-carbon chain with five hydroxyl groups positioned similarly to the open-chained form of glucose and terminating in a carboxylic acid group. Gluconic acid is in equilibrium with the cyclic ester glucono delta-lactone in aqueous solution. It is a mild organic acid produced by a simple oxidation reaction from glucose.
Production
Gluconic acid is made by fermenting glucose and producing the physiological d-form. Hlasiwetz and Habermann reported on its preparation in 1870, which involved the chemical oxidation of glucose. Boutroux used glucose fermentation to prepare and isolate gluconic acid in 1880.
Modern gluconic acid production methods rely on variations of glucose (or other carbohydrate-containing substrate) oxidation via fermentation or noble metal catalysis. Because it is a polyhydroxycarboxylic acid with both hydroxyl and carboxyl groups that can react, it has versatile properties.
Occurrence and applications
Gluconic acid is found in fruits, honey, and wine. It is now known as an acidity regulator as a food additive. Ca2+, Fe2+, Al3+, and other metals, including lanthanides and actinides, are chelated by the gluconate anion. It is also used in cleaning products to dissolve mineral deposits, particularly in alkaline solutions.
Calcium gluconate gel is used to treat hydrofluoric acid burns; calcium gluconate injections may be used in more severe cases to avoid necrosis of deep tissues and to treat hypocalcemia in hospitalized patients. Gluconate is also an electrolyte found in some solutions, such as “plasmalyte a,” which is used to resuscitate intravenous fluids. Quinine gluconate is a salt of gluconic acid and quinine, which is used for intramuscular injection in the treatment of malaria.