The body will heal given enough time and energy, but when doctors or engineers intervene, the processes do not always go as planned because chemicals that control and facilitate the healing process are missing. Now, an international team of engineers is bioprinting bone along with two growth factor encoding genes that aid in cell incorporation and the healing of defects in rats’ skulls.
“Growth factors are critical for cell growth,” explained Ibrahim T. Ozbolat, associate professor of engineering science and mechanics. “We use two distinct genes that encode two distinct growth factors. These growth factors aid in the migration of stem cells into the defect area and the conversion of progenitor cells into bone.”
The researchers used gene encoding PDGF-B, platelet derived-growth factor, which encourages cells to multiply and to migrate, and gene encoding BMP-2, bone morphogenetic protein, which improves bone regeneration. They delivered both genes using bioprinting.
“We used a controlled co-delivery release of plasmids from a gene-activated matrix to promote bone repair,” the researchers stated in the journal Biomaterials.
Growth factors are critical for cell growth. We use two distinct genes that encode two distinct growth factors. These growth factors aid in the migration of stem cells into the defect area and the conversion of progenitor cells into bone.
T. Ozbolat
Ozbolat and his colleagues embedded the protein’s DNA in plasmids, which are ringlike loops of DNA that can transport genetic information. When the DNA enters the progenitor cell, it begins to produce the proteins needed to promote bone growth.
During surgery, the two genes were printed onto a hole in the skull of a rat using a device similar to an ink-jet printer. The mixture was designed to release a burst of PDGF-B encoding gene in 10 days and a continuous release of BMP-2 encoding gene over the course of five weeks.
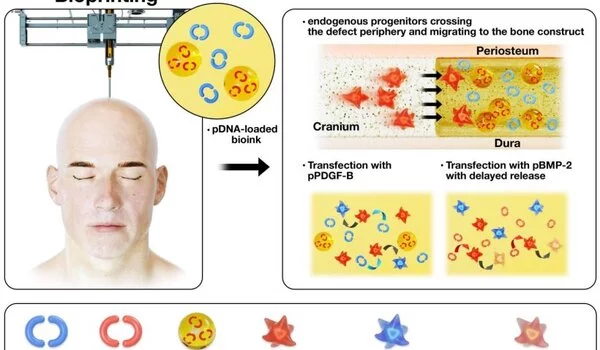
The rats that received bioprinted genes with controlled release of BMP-2 encoding gene saw about 40% bone tissue creation and 90% bone coverage in six weeks compared to 10% new bone tissue and 25% bone coverage for rats with the same defect, but no treatment.
“This method is better than simply dumping the growth factors,” said Ozbolat. “If we do that, the amounts of proteins are finite, but if we use gene therapy, the cells continue to produce the necessary growth factors.”
Working with Ozbolat from Penn State were Kazim K. Moncal, graduate student in engineering science and mechanics; Gregory S. Lewis, assistant professor and Hwabok Wee, postdoctoral fellow both in orthopedics and rehabilitation; Kevin P. Godzik, undergraduate in biomedical engineering: and Elias Rizk, associate professor of neurosurgery.
R. Seda Tigli Aydin, former Penn State postdoctoral fellow now at Bulen Ecevit University in Turkey; Dong N. Heo, former Penn State postdoctoral fellow now at Kyung Hee University in South Korea; and Timothy M. Acri, former graduate researcher and Aliasger K. Salem, Lyle and Sharon Bighley Endowed Chair & Professor in Pharmaceutical Sciences, University of Iowa, also contributed to the research.
This research was funded by the International Team for Implantology, the National Institutes of Health, the National Science Foundation, the Osteology Foundation, and the Turkish Scientific and Technological Research Council.