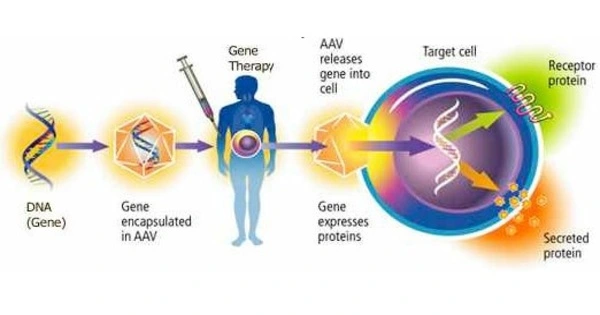
Gene therapy for glioblastoma frequently seeks to selectively deliver therapeutic genes or chemicals to malignant cells while protecting healthy brain tissue. To influence gene expression, one way is to use specific promoters that are active in glioblastoma cells.
Glioblastoma (GBM), an aggressive brain cancer, is famously resistant to therapy, with recurrent GBM associated with a 10-month survival rate. Immunotherapies, which activate the body’s immune defenses against cancer, have not been helpful for GBM, in part because the tumor’s surrounding environment is mostly resistant to immune system assaults.
Researchers from Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, engineered a novel oncolytic virus that can infect cancer cells and stimulate an anti-tumor immune response to convert this immunosuppressive environment into one amenable to an immune response. The findings, published in Nature, indicated the innovative gene therapy approach’s safety and preliminary efficacy in high-grade glioma patients, with prolonged survival in a subset of recurrent GBM patients immunologically “familiar” with the virus.
“GBM has an aggressive effect in part because of a milieu of immunosuppressive factors surrounding the tumor, which enable the tumor’s growth by preventing the immune system from entering and attacking it,” said corresponding author E. Antonio Chiocca, MD, PhD, Chair of the BWH Department of Neurosurgery. “This study showed that with a virus we designed, we can reshape this ‘immune desert’ into a pro-inflammatory environment.”
GBM has an aggressive effect in part because of a milieu of immunosuppressive factors surrounding the tumor, which enable the tumor’s growth by preventing the immune system from entering and attacking it. This study showed that with a virus we designed, we can reshape this ‘immune desert’ into a pro-inflammatory environment.
E. Antonio Chiocca
This phase I, the first-in-human trial examined the safety of an oncolytic virus, called CAN-3110, which was designed and subjected to preclinical testing by researchers at BWH and licensed to Candel Therapeutics as the trial was ongoing.
The cancer-causing virus is an oncolytic herpes simplex virus (oHSV), which is the same type of virus utilized in a therapy approved to treat metastatic melanoma. This therapy, unlike other clinical oHSVs, has the ICP34.5 gene, which is frequently excluded from clinical oHSVs since it causes human disease in unaltered forms of the virus. However, the researchers suggested that this gene may be required to initiate a powerful, pro-inflammatory response required for tumor assault. As a result, they created an oHSV1 variant that retains the ICP34.5 gene but is also genetically “programmed” not to assault healthy brain cells.
Overall, the research showed that CAN-3110 was safe in 41 patients with high-grade gliomas, including 32 with recurrent GBM. Seizures were the most serious adverse events in two subjects. Notably, GBM patients with pre-existing HSV1 viral antibodies (66% of the patients) had a median overall survival of 14.2 months. The researchers discovered signs of multiple alterations in the tumor microenvironment related with immune activation in patients who already had antibodies. They suggest that the presence of HSV1 antibodies led in a rapid immunological response to the virus, bringing more immune cells to the tumor and increasing inflammation levels in the tumor microenvironment.
The researchers also detected an increase in the diversity of the T cell repertoire after CAN-3110 therapy, implying that the virus produces a wide immune response, possibly by destroying tumor cells, resulting in the release of cancer antigens. These immunological alterations following treatment have also been linked to increased survival.
Studies like this one demonstrate gene therapy’s potential for addressing difficult-to-treat illnesses. The Gene and Cell Therapy Institute at Mass General Brigham is assisting in the translation of scientific discoveries generated by researchers into first-in-human clinical trials and, ultimately, life-changing treatments for patients. The comprehensive approach of the Institute distinguishes it from others in the field, allowing researchers to swiftly advance innovative therapies and push the technological and clinical frontiers of this new frontier.
The researchers intend to conduct prospective studies to further evaluate the efficiency of the oncolytic virus in patients with and without HSV1 antibodies in the future. After demonstrating the safety of a single viral injection, they are now testing the safety and efficacy of up to six injections over four months, which, like numerous rounds of vaccination, may boost the therapy’s effectiveness. Break Through Cancer is funding the new six-injection experiment.
“Almost no immunotherapies for GBM have been able to increase immune infiltration to these tumors, but the virus studied here provoked a very reactive immune response with infiltration of tumor-killing T-cells,” Chiocca said in a press release. “That’s hard to do with GBM, so our findings are exciting and give us hope for our next steps.”