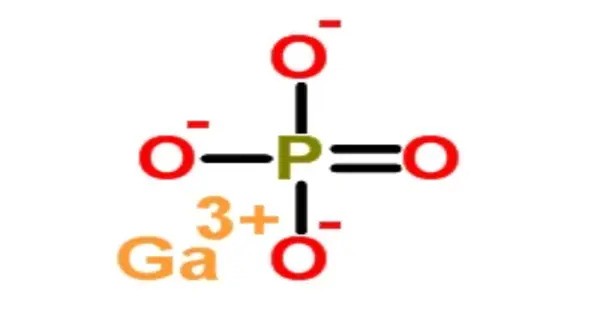
Gallium phosphate (GaPO4 or gallium orthophosphate) is a colorless trigonal crystal with a hardness of 5.5 on the Mohs scale. GaPO4 is isotypic with quartz, possessing very similar properties, but the silicon atoms are alternately substituted with gallium and phosphorus, thereby doubling the piezoelectric effect. GaPO4 has many advantages over quartz for technical applications, like a higher electromechanical coupling coefficient in resonators, due to this doubling. Contrary to quartz, GaPO4 is not found in nature. Therefore, a hydrothermal process must be used to synthesize the crystal.
GaPO₄ exhibits exceptional piezoelectric performance, surpassing quartz in sensitivity and stability, which makes it ideal for sensors, resonators, and ultrasonic devices. Its wide bandgap and transparency in the ultraviolet to infrared range also enable applications in optics, such as lenses and laser components. Additionally, gallium phosphate is explored in catalysis and as a substrate for semiconductor growth due to its lattice compatibility with gallium-based compounds.
Properties
Chemically stable and insoluble in water, it resembles aluminum phosphate and is often synthesized via hydrothermal methods or high-temperature reactions. Its high thermal stability, with a melting point above 1,670°C, makes it suitable for high-temperature applications.
- Chemical formula: GaPO4
- Molar mass: 164.694 g/mol
- Appearance: Transparent crystals
- Solubility in water: Insoluble
- Refractive index (nD): no=1.605, ne=1.623
Modifications
GaPO4 possesses, in contrast to quartz, no α-β phase transition, thus the low temperature structure (structure like α-quartz) of GaPO4 is stable up to 970°C, as are most of its other physical properties. Around 970°C another phase transition occurs which changes the low quartz structure into another structure similar with cristobalite.
Structure
The specific structure of GaPO4 shows the arrangement of tetrahedrons consisting of GaO4 and PO4 that are slightly tilted. Because of the helical arrangement of these tetrahedrons, two modifications of GaPO4 exist with different optical rotation (left and right).
Sources
GaPO4 does not occur in nature; thus it must be grown synthetically. Presently, only one company in Austria produces these crystals commercially. Found naturally in trace amounts, it is primarily produced synthetically for industrial use. Its low dielectric loss and high mechanical quality factor enhance its utility in precise frequency control devices, like oscillators in telecommunications. Research continues into its potential in nanotechnology and energy harvesting, leveraging its robust chemical and physical properties for advanced applications.
Natural Occurrence
- Gallium is a trace element, typically found in bauxite ores (aluminum ores) and zinc ores, but not as GaPO₄.
- Naturally occurring phosphates are more commonly associated with minerals like apatite or monazite, which do not typically contain gallium.
Synthetic Production
- GaPO₄ is synthesized via hydrothermal methods or high-temperature chemical processes, mimicking conditions used to grow synthetic quartz.
- Gallium is extracted from bauxite or as a byproduct of zinc refining, then reacted with phosphoric acid or phosphate precursors to form GaPO₄ crystals.
Applications
- Used in piezoelectric devices, such as high-precision resonators, filters, and sensors for automotive, aerospace, and industrial applications.
- Employed in high-temperature pressure sensors due to its thermal stability.
- Explored for use in microelectromechanical systems (MEMS) and optical devices.