Fuel Cell
Definition
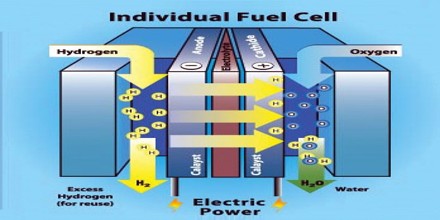
Fuel cell is like a battery in that it generates electricity from an electrochemical reaction. Both batteries and fuel cells convert chemical potential energy into electrical energy and also, as a by-product of this process, into heat energy. A fuel cell produces electricity through a chemical reaction, but without combustion. It converts hydrogen and oxygen into water and in the process also creates electricity. It’s an electro-chemical energy conversion device that produces electricity, water, and heat.
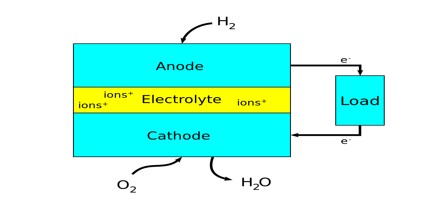
It is a device that produces electricity by combining a fuel, usually hydrogen, with oxygen. Every fuel cell has two electrodes called, respectively, the anode and cathode. The reactions that produce electricity take place at the electrodes.
Hydrogen is the basic fuel, but fuel cells also require oxygen. One great appeal of fuel cells is that they generate electricity with very little pollution–much of the hydrogen and oxygen used in generating electricity ultimately combines to form a harmless byproduct, namely water. Fuel cells are currently used in spacecraft, and increasingly in ground transportation, with potential use everywhere electricity is required.
Types and Functions of Fuel Cell
Fuel cells are generally classified according to the nature of the electrolyte, each type requiring particular materials and fuel. Each fuel cell type also has its own operational characteristics, offering advantages to particular applications. This makes fuel cells a very versatile technology.
Polymer Electrolyte Membrane (PEM) fuel cells—also called proton exchange membrane fuel cells, which is deliver high power density and offer the advantages of low weight and volume compared with other fuel cells. PEM fuel cells use a solid polymer as an electrolyte and porous carbon electrodes containing a platinum or platinum alloy catalyst.
Direct Methanol Fuel Cells (DMFCs) – Direct methanol fuel cells, however, are powered by pure methanol, which is usually mixed with water and fed directly to the fuel cell anode. DMFCs are often used to provide power for portable fuel cell applications such as cell phones or laptop computers.
Phosphoric Acid Fuel Cells (PAFCs) – Phosphoric acid fuel cells use liquid phosphoric acid as an electrolyte the acid is contained in a Teflon-bonded silicon carbide matrix and porous carbon electrodes containing a platinum catalyst. This type of fuel cell is typically used for stationary power generation, but some PAFCs have been used to power large vehicles such as city buses.
Solid Oxide Fuel Cells (SOFCs) – Solid oxide fuel cells use a hard, non-porous ceramic compound as the electrolyte. SOFCs are around 60% efficient at converting fuel to electricity. In applications designed to capture and utilize the system’s waste heat (co-generation), overall fuel use efficiencies could top 85%.
Molten Carbonate Fuel Cells (MCFCs) – Molten carbonate fuel cells are currently being developed for natural gas and coal-based power plants for electrical utility, industrial, and military applications. MCFCs are high-temperature fuel cells that use an electrolyte composed of a molten carbonate salt mixture suspended in a porous, chemically inert ceramic lithium aluminum oxide matrix.
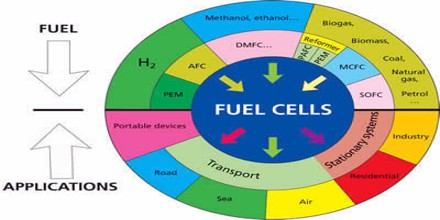
Applications of Fuel Cell
Fuel cells can be used for many transportation applications including automobiles, buses, utility vehicles, and scooters and bicycles. Many fuel cell demonstration vehicles have been created for each of these vehicle types. Stationary fuel cells are used for commercial, industrial and residential primary and backup power generation. Fuel cells are very useful as power sources in remote locations, such as spacecraft, remote weather stations, large parks, communications centers, rural locations including research stations, and in certain military applications.
Molten Carbonate fuel cells (MCFC) use high-temperature compounds of salt (like sodium or magnesium) carbonates (chemically, CO3) as the electrolyte. The high temperature limits damage from carbon monoxide “poisoning” of the cell and waste heat can be recycled to make additional electricity.
Portable power systems that use fuel cells can be used in the leisure sector, the industrial sector, and in the military sector. SFC Energy is a German manufacturer of direct methanol fuel cells for a variety of portable power systems.
Reference: fuelcelltoday.com, energy.gov, fuelcellstore.com, wikipedia.