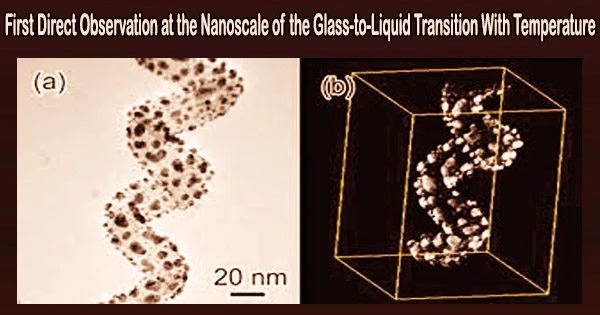
In order to examine the “glass transition,” which occurs when glass is heated and transforms into a supercooled liquid phase, under the microscope in real time for the first time, researchers from the UAB and the ICN2 have created a methodology.
The study, which was published in Nature Physics, has significant implications for the cryopreservation of proteins, cells, and living tissues as well as for the production of medicines, electronics, and tissue engineering, all of which depend on the glass-to-liquid transition.
Glass is a solid substance with a structure so disorganized that it could be compared to a very viscous liquid. It is present in fiber optics, industrial plastic materials, television screens, mobile devices, windows that are clear or stained, windows and stained glass, as well as the frozen state of proteins, cellular structures, and living tissues used for cryopreservation.
Despite being so widespread, it is quite challenging to create theories and models that can thoroughly explain their behavior. The methods by which a liquid cools and changes into a glass, and in the opposite direction, how a glass changes into a liquid when heated, a phenomenon known as “glass transition,” are still poorly understood.
Physicists are still unsure of whether this is a phase transition, making glass a distinct thermodynamic state from liquid and solid states, or whether glass is simply a supercooled liquid that has been cooled below freezing but has retained its liquid properties and has very little mobility of its atoms or molecules.
The microscopic description we have achieved has made possible for the first time a direct comparison between computational models and physical reality. We believe that this technique will also be very useful in exploring the glass transition on smaller time and space scales, which will allow a better understanding of the transition in less stable glass produced from cooled liquids.
Javier Rodríguez Viejo
Since the microstructure of the supercooled liquid and glass are nearly identical, one of the main hurdles in understanding this process is the difficulty in observing it with adequate clarity under the microscope.
A team led by researchers from the Department of Physics of the Universitat Autònoma de Barcelona (UAB) and the Catalan Institute of Nanoscience and Nanotechnology (ICN2), with the involvement of the UPC and the IMB-CNM-CSIC, has presented a new methodology that makes it possible to observe directly under the microscope what happens in a glass when it is heated above the glass transition temperature, known as the “relaxation” process that transforms it into a liquid.
The use of thermal evaporation to create ultra-stable organic glass was a research tool. They are more stable kinetically and thermodynamically than traditional glass made straight from liquids, and they are denser.
This ultra-stable glass transitions to a supercooled liquid state similarly to how crystalline solids do when they transition to the liquid state, with the formation of liquid-phase areas that grow steadily larger. This is in contrast to conventional glass, which, as has been observed thus far, transforms to the liquid state globally, without obvious distinctions between different regions of the material.
This is a phenomenon that was previously only indirectly observed in computer models and explained by nanocalorimetry experiments.
“Previously it had already been inferred from these models that the liquid-phase areas that are produced have an extraordinary separation between them when it comes to ultra-stable glass, but this had never been observed directly,” says Cristian Rodriguez Tinoco, researcher at the UAB and ICN2.
Sandwiching the ultra-stable glass between two layers of glass with a higher transition temperature is the innovative technique created to witness this transition. The instabilities that develop on the surface are transported to the sandwich’s outer layers when the ultrastable glass layer is heated over its transition temperature, and they are then visible with an atomic force microscope.
“These are very small movements and compressions, of the order of a few nanometers when the transformation begins, but large enough to be measured precisely with a microscope of this type, which monitors in situ the surface deformations that appear above the transition temperature,” explains Ph.D. student Marta Ruiz Ruiz.
The work allows the devitrification of the glass to be followed in real time. It enables for the direct measurement of the distances between the liquid domains that develop, while also watching the deformation of the surface and its evolution over time, of the relaxation process in ultra-stable crystals toward a supercooled liquid.
In this approach, it was feasible to confirm that the distances between liquid areas in this type of glass are exceptionally enormous and that they correlate with the material’s time scales, as predicted by computational models.
“The microscopic description we have achieved has made possible for the first time a direct comparison between computational models and physical reality. We believe that this technique will also be very useful in exploring the glass transition on smaller time and space scales, which will allow a better understanding of the transition in less stable glass produced from cooled liquids,” concludes Javier Rodríguez Viejo, researcher at the UAB and ICN2.