Electricity is everywhere in our lives. From the voltage that comes from the wall to power our lights, to the batteries in our phones and computers that keep us connected on the go, electricity is essential to modern society. The energy we get from batteries actually comes from chemical energy. In a battery, the chemical energy is converted into electrical energy.
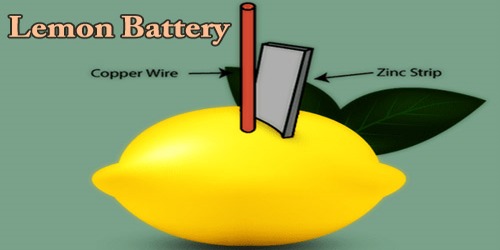
A lemon battery is a simple battery made using a zinc metal like a galvanized nail and a copper piece like a penny for educational purposes. These are inserted into a lemon and are connected by wires. The zinc and copper are called electrodes and lemon juice is an electrolyte.
The lemon battery is similar to the first electrical battery invented in 1800 by Alessandro Volta, who used brine (saltwater) instead of lemon juice. The lemon battery illustrates the type of chemical reaction (oxidation-reduction) that occurs in batteries. The zinc and copper are called the electrodes, and the juice inside the lemon is called the electrolyte. There are many variations of the lemon cell that use different fruits (or liquids) as electrolytes and metals other than zinc and copper as electrodes.
Many fruits and liquids can be used for the acidic electrolyte. The fruit is convenient because it provides both the electrolyte and a simple way to support the electrodes. The acid involved in citrus fruits (lemons, oranges, grapefruits, etc.) is citric acid. The acidity, which is indicated by the measured pH, varies substantially.
The functioning of a Lemon Battery –
Things we will need:
- Citrus fruits (Lemon will be best)
- Copper wire of 18 gauge or lesser
- Wirecutter/stripper
- A Zinc piece, small nail, Steel clip as an electrical conductor

Procedure:
- Strip around two and a half inches of plastic off the wire and cut that section away from the roll of copper wire we have.
- Take the paper clip and straighten it out. Using the clippers cut it to the same length as the wire of copper.
- Rub off any of the rough spots that are on our conductors. The end of the wire must be smooth as we will touch that to our tongue.
- The lemon needs to be pressed so that its cell walls breakdown and the juice is released. For the chemical reaction to take place the sour juices of lemon should be properly released.
- Take the copper wire and stick it for about an inch inside the lemon.
- Take the two free ends and stick them to our tongue.
When we touched our tongue to just the copper wire, we most likely would not have noticed anything unusual. When we touched our tongue to BOTH of the metal ends, we might have felt a tingle or noticed a metallic taste. The tingle or metal taste we noticed shows that our lemon battery was generating an electric current. That means tiny electrons were moving across the surface of our tongue. Electrons are subatomic particles that zoom around an atom’s center and make up the part of the atom that is negatively charged.
The lemon battery we made is a type of battery called a voltaic battery. These types of batteries are made of two different metals, which act as electrodes, or places where electrons can enter or leave a battery. In our case, the electrical current entered our tongue, which is why we felt a tingle.
So why were we able to stick electrodes into a lemon and get a battery? All voltaic batteries need their metals to be placed in an electrolyte. An electrolyte is a substance that can carry electrical current when dissolved in water. The tiny bit of salt in our saliva makes our saliva an electrolyte, and the sour citric acid does the same thing for lemon juice. Batteries stop working when there is not enough of the electrolyte to react with the metal or not enough metal left to react with the electrolyte.
Instead of fruit, liquids in various containers can be used. Household vinegar (acetic acid) works well. Sauerkraut (lactic acid) was featured in one episode of the US television program Head Rush (an offshoot of the MythBusters program). The sauerkraut had been canned and became the electrolyte while the can itself was one of the electrodes.
Zinc and copper electrodes are reasonably safe and easy to obtain. Other metals such as lead, iron, magnesium, etc., can be studied as well; they yield different voltages than the zinc/copper pair. In particular, magnesium/copper cells can generate voltages as large as 1.6 V in lemon cells. This voltage is larger than obtainable using zinc/copper cells. It is comparable to that of standard household batteries (1.5 V), which is useful in powering devices with a single cell instead of using cells in series.
Information Sources: