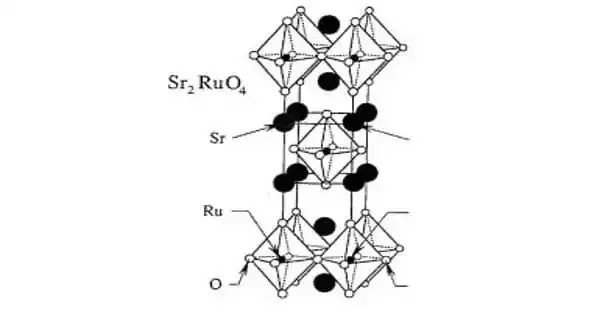
Distrontium ruthenate is a fascinating perovskite superconductor, notable as the first copper-free oxide to exhibit superconductivity, discovered by Yoshiteru Maeno in 1994. It is also known as strontium ruthenate, is an oxide of strontium and ruthenium with the chemical formula Sr2RuO4. It was the first reported perovskite superconductor that did not contain copper. It has a layered crystal structure similar to high-temperature cuprate superconductors like (La,Sr)₂CuO₄, but its superconducting transition occurs at a much lower temperature of 0.93 K (up to 1.5 K in optimal samples).
Strontium ruthenate is structurally very similar to the high-temperature cuprate superconductors, and in particular, is almost identical to the lanthanum doped superconductor (La, Sr)2CuO4. However, the transition temperature for the superconducting phase transition is 0.93 K (about 1.5 K for the best sample), which is much lower than the corresponding value for cuprates.
Properties
- Appearance: Typically synthesized as high-quality single crystals, often black or dark in color due to its metallic nature.
- Stability: Stable under controlled conditions but sensitive to synthesis parameters. It can undergo potential-induced transformations affecting stability, particularly in thin films.
- Magnetic Properties: Unlike its ferromagnetic cousin SrRuO₃, Sr₂RuO₄ is not ferromagnetic but shows complex magnetic behavior linked to its unconventional superconductivity.
Structure
Unlike cuprates, it achieves superconductivity without doping and displays unconventional p-wave pairing, potentially linked to a Fulde–Ferrell–Larkin–Ovchinnikov phase. Its tetragonal structure (space group I4/mmm) and Fermi liquid behavior below 25 K make it a model system for studying exotic quantum states. Recent studies show its critical temperature can increase under uniaxial strain near a Van Hove singularity, revealing insights into its pairing symmetry via the elastocaloric effect.
Synthesis
Sr₂RuO₄ is synthesized through methods like floating-zone melting, producing high-quality crystals for research. Its unique properties, including time-reversal symmetry breaking, make it a key material for exploring topological quantum states and unconventional superconductivity, with applications in advanced electronics and quantum devices.
Superconductivity
Superconductivity in SRO was first observed by Yoshiteru Maeno et al. Unlike the cuprate superconductors, SRO displays superconductivity in the absence of doping. The superconducting order parameter in SRO exhibits signatures of time-reversal symmetry breaking, and hence, it can be classified as an unconventional superconductor.
Occurrences
- Natural Occurrence: Sr₂RuO₄ is not found naturally in significant quantities. Ruthenium, a key component, occurs as a minor constituent in platinum group metal ores, primarily in the Ural Mountains, North and South America, and in pentlandite from Sudbury, Ontario, or pyroxenite deposits in South Africa. However, Sr₂RuO₄ itself is a synthetic compound.
- Synthetic Production: Produced in laboratories for research, particularly for studying unconventional superconductivity and related phenomena. Its rarity in nature and complex synthesis make it primarily a research material rather than a naturally occurring mineral.