Dinickel boride is a chemical compound of nickel and boron with the formula Ni2B. It is an efficient catalyst and reducing agent. It is one of the borides of nickel. It is used as a heterogeneous hydrogenation catalyst.
The formula “Ni2B” and the name “nickel boride” are often used for a nickel-boron catalyst obtained by reacting nickel salts with sodium borohydride. Nickel catalysts are designed for steam reforming hydrocarbons and methane. However, that product is not a well-defined compound, and its bulk formula is closer to Na2.5B.
Structure
Ni2B has been suggested to be an amorphous compound, composed of nickel bonded to individual boron centers. However, it contains nanoparticles of nickel which on heating under inert conditions become more crystalline.
Properties
Nickel boride is in the form of black amorphous powder or black granules. It is insoluble in all solvents but reacts with concentrated mineral acids. The solid is air-stable. As expected for a boride, it has a high melting point.
Synthesis
Dinickel boride can be obtained (together with other nickel borides) by heating sodium borohydride with powdered nickel-metal up to 670 °C in a closed vessel so that the released hydrogen creates a pressure of up to 3.4 MPa. The main reactions can be summarized as
2NaBH4 ↔ 2NaH + B2H6
2Ni + 2B2H6 + NaH ↔ Ni2B + 3BH3 + 2H2 + Na
but other reactions occur, yielding other borides.
Preparation
The preparation of amorphous nickel boride is simple compared with other borides which requires high temperatures, special techniques and equipment.
The P−1 form of Ni2B can be generated by mixing nickel(II) sulfate and sodium borohydride in alkaline aqueous solutions. The product is not nickel boride but nanoparticles of nickel disperse in a boron compound matrix. The P−2 form is prepared similarly from nickel(II) acetate and sodium borohydride in ethanol. The product precipitates as a fine, black amorphous powder.
Safety
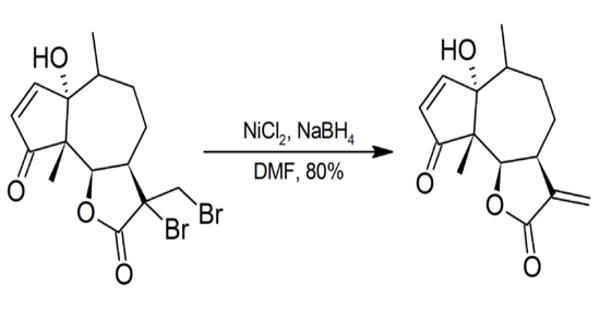
Nickel compounds are possible carcinogens and contact with the skin should be avoided. Particular care should be taken whenever NiCl2/NaBH4 is used in DMF as sodium borohydride may spontaneously ignite in DMF.
Information Source: