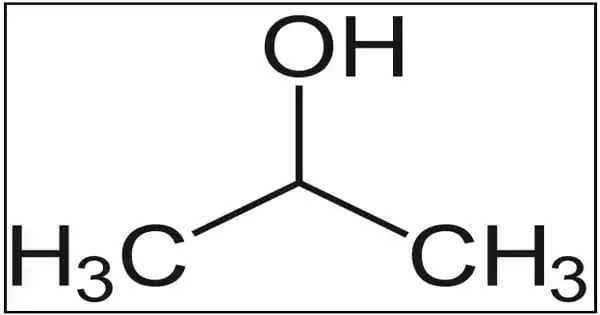
Denatured alcohol is ethanol that has been treated with additives to make it poisonous, bad-tasting, foul-smelling, or nauseating in order to discourage its recreational use. It is ethyl alcohol with toxic or unpleasant additives that render it unfit for human consumption. It is sometimes dyed to help identify it visually. 5 to 10% methanol is the most common additive to denatured alcohol. When consumed orally, methanol is extremely toxic. Denatured alcohol is poisonous when contaminated with pyridine or methanol, and it is bitter when contaminated with denatonium. This type of ethanol has a bad taste, a foul odor, and is poisonous if consumed.
Denatured alcohol is used as a solvent as well as a fuel source for alcohol burners and camping stoves. It is an alcohol to which enough malodorous and obnoxious substances have been added to prevent its use or recovery for beverage purposes but not for many industrial uses. Hundreds of additives and denaturing methods have been used due to the wide range of industrial applications for denatured alcohol. The main additive is typically 10% methanol (methyl alcohol), thus the name methylated spirits. Isopropyl alcohol, acetone, methyl ethyl ketone, and methyl isobutyl ketone are some other common additives.
Unlike in biochemistry, denaturing alcohol has no effect on the ethanol molecule (chemically or structurally). Rather, the ethanol is combined with other chemicals to create a foul-tasting, frequently toxic solution. Many of these solutions make it difficult to separate the components on purpose.
Uses
Except for applications involving fuel, surgical supplies, and laboratory stock, denatured alcohol is used in the same way as ethanol. It is used to sand wood, as a fuel for small camping stoves, as a cleaning aid, and as a solvent, among other things. It kills germs, which is why it’s used in hand sanitizers and cleaning products, and it’s highly flammable, making it ideal for camping stove fuel.
Pure ethanol is required for food and beverage applications, as well as certain chemical reactions that would be hampered by the denaturant. Denatured ethanol cannot be used to precipitate nucleic acids in molecular biology.
Denatured alcohol has no advantages over regular ethanol for any purpose; it is a public policy compromise. Because denatured alcohol is sold without the often high taxes on alcohol suitable for consumption, it is a less expensive solution for most non-drinking applications. If pure ethanol could be made inexpensively available for use as a fuel, solvent, or medicine, it would likely be enjoyed as a drink by a large number of people without the payment of alcoholic beverage taxes.
Toxicity
Despite its poisonous nature, denatured alcohol is occasionally consumed as a substitute for alcohol. If it contains methanol, this can cause blindness or death. During Prohibition in the United States, for example, federal law required methanol in domestically manufactured industrial alcohols. Denatonium, which has an extremely bitter taste, is frequently added to help prevent this. To give the mixture an unpleasant odor, substances such as pyridine are added, and agents such as ipecac syrup may also be included to induce vomiting.