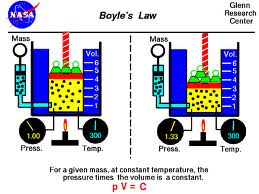
This article focus to Define and Discuss on Boyle’s Law. English scientist Robert Boyle performed a number of experiments involving pressure, in 1662, arrived for a general law—that the number of a gas varies inversely having pressure. Pressure is the volume of force exerted on one unit of area. The example associated with an ocean diver should make the style clearer: The greater the depth the diver actually reaches, the greater the pressure due to the weight of the overlying normal water.
Define and Discuss on Boyle’s Law