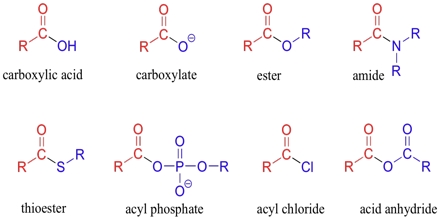
Basic objective of this lecture is to discuss on Carboxylic Acids and their derivatives. Here also Physical properties of alcohols Molecules have attractive forces between the molecules called hydrogen bonds, not as strong as covalent bonds. Finally discuss on physical properties of ethers. In sther, no hydrogen on the oxygen atoms to form hydrogen bonds – only weak forces of attraction between molecules. Boiling points similar to corresponding alkane.
Carboxylic Acids