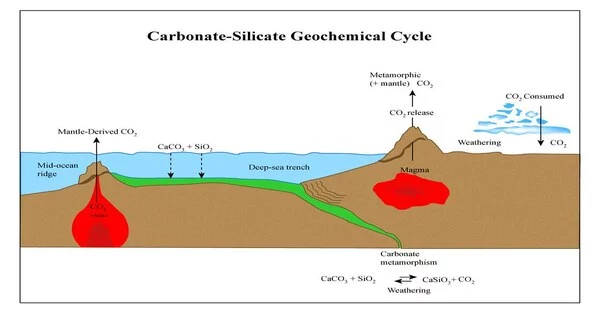
The carbonate–silicate geochemical cycle is a natural process in which carbon dioxide (CO2) is removed from the Earth’s atmosphere and converted into carbonates through chemical reactions with silicate rocks. This cycle plays an important role in regulating the Earth’s climate over long periods of time, as it acts as a long-term carbon sink.
The cycle begins with the weathering of silicate rocks on the Earth’s surface, which releases ions such as calcium, magnesium, and bicarbonate into the surrounding soil and water. These ions are carried by water and eventually reach the ocean, where they react with dissolved carbon dioxide to form calcium carbonate (CaCO3) and other carbonates. This process is called mineral carbonation.
The carbonate-silicate geochemical cycle, also known as the inorganic carbon cycle, describes the long-term transformation of silicate rocks into carbonate rocks through weathering and sedimentation, as well as the transformation of carbonate rocks back into silicate rocks through metamorphism and volcanism. The carbon dioxide in the atmosphere is removed from the atmosphere during the burial of weathered minerals and returned to the atmosphere via volcanism. On million-year time scales, the carbonate-silicate cycle is an important factor in climate control because it regulates carbon dioxide levels and thus global temperature.
Over time, the carbonates accumulate in the ocean and can be deposited on the seafloor as sediments. Some of these sediments may become buried deep within the Earth’s crust and undergo metamorphism, where they are subjected to high pressure and temperature. This process can cause the carbonates to release their carbon dioxide back into the atmosphere through volcanic eruptions or other geologic activity.
However, the rate of weathering is affected by factors such as how much land is exposed. These factors include changes in sea level, topography, lithology, and vegetation. Furthermore, geomorphic and chemical changes have worked in tandem with solar forcing to determine global surface temperature, whether due to orbital changes or stellar evolution. Furthermore, the carbonate-silicate cycle has been proposed as a possible solution to the paradox of the faint young Sun.
The overall effect of the carbonate–silicate geochemical cycle is to regulate the amount of carbon dioxide in the atmosphere over long periods of time. By removing carbon dioxide from the atmosphere and storing it in the form of carbonates, the cycle helps to prevent the Earth from becoming too warm or too cold for life to thrive. However, the process is slow and takes millions of years to complete, which means that it is not effective at mitigating the short-term effects of human-caused carbon emissions.