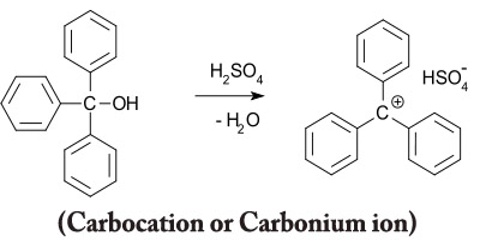
Carbocation or Carbonium ion
All carbocations also known as carbonium ions carry a positive charge on a carbon atom. The name tells you that – a cation is a positive ion, and the “carbo” bit refers to a carbon atom. However there are important differences in the structures of various types of carbocations.
Some university-level textbooks discuss carbocations as if they were only carbenium ions, or discuss carbocations with only a fleeting reference to the older terminology of carbonium ions or carbenium and carbonium ions. One textbook retains the older name of carbonium ion for carbenium ion to this day, and uses the phrase hypervalent carbenium ion for CH+5.
The orbitals of carbocations are generally sp2 hybridized so that the three full orbitals are arranged in a trigonal planar geometry about the carbon nucleus. The remaining p orbital is empty and will readily accept a pair of electrons from another atom. Because of the symmetry of this geometric arrangement, nucleophilic attack is equally favorable above or below the plane formed by the full orbitals.
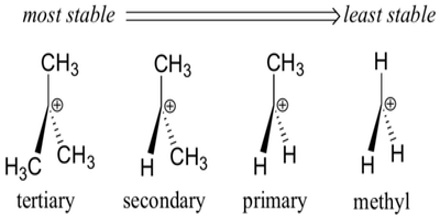
Carbocation Classification
In order to understand carbocations, we need to learn some basic carbocation nomenclature. A primary carbocation is one in which there is one carbon group attached to the carbon bearing the positive charge. A secondary carbocation is one in which there are two carbons attached to the carbon bearing the positive charge. Likewise, a tertiary carbocation is one in which there are three carbons attached to the carbon bearing the positive charge.
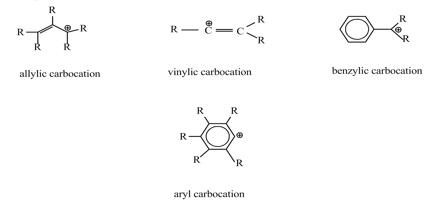
If the carbon bearing the positive charge is immediately adjacent to a carbon-carbon double bond, the carbocation is termed an allylic carbocation.
Carbocation Stability
The stability of carbocations is dependent on a few factors. The first factor to look at when deciding the stability of a carbocation is resonance. Resonance is a stabilizing feature to a carbocation because it delocalizes the positive charge and creates additional bonding between adjacent atoms. Decreasing the electron deficiency increases the stability.

Carbocations can also be stabilized through resonance by neighboring lone pairs or pi-electrons. In general, this stabilization is greater than one degree of substitution, so a secondary carbocation stabilized by resonance will be more stable than a tertiary carbocation with no resonance stabilization, and a primary carbocation stabilized by resonance will be more stable than a secondary carbocation with no resonance stabilization.
==Formation of Carbocations==y Carbocation intermediates are formed in three main types of reactions: additions to pi bonds, unimolecular eliminations, and unimolecular nucleophilic substitution. On a bridge head a positive carbon is rare. The 3-cyclopropyl carbocation is the most stable carbocation.
Three Fates of a Carbocation
Now we consider how carbocations behave in reaction mechanisms. Generally speaking, carbocations are unstable due to their open octets and positive charges. Thus, their reactions will be strongly influenced by filling the octet of the carbon bearing the positive charge, or at least making this positive charge more stable. There are three common mechanism pathways (or fates) by which carbocations may achieve this stability. These fates are (a) capture a nucleophile, (b) lose a proton to form a π bond, and (c) rearrange.
Capture a Nucleophile. The carbocation is electrophilic because it has a positive charge and (in most cases) a carbon atom with an open octet. The positive charge is neutralized when an electron pair is accepted and a new covalent bond is formed. By definition, a species that donates a pair of electrons to form a new covalent bond is a nucleophile.
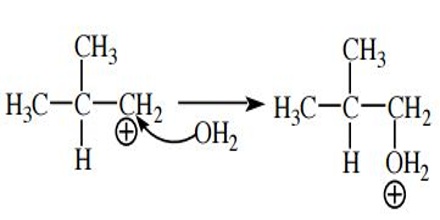
Because carbocations are very reactive, even weak nucleophiles such as water can be captured with ease.
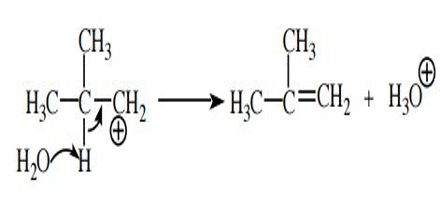
Lose a proton to form a π bond. Accepting an electron pair from an adjacent bond to a hydrogen atom neutralizes the positive charge or fills the open octet and forms a new π bond. (The carbocation carbon now has four bonds and a full octet, so its formal charge is zero.) The hydrogen atom must be removed by a base, but because carbocations are generally very reactive species and very strongly driven to dispose of the positive charge even a weak base such as water or iodide ion can accomplish this deprotonation.
Rearrangement. The bonding electrons of a carbocation may shift between adjacent atoms to form a more stable carbocation. For example, rearrangement will occur if a secondary carbocation can be formed from a primary carbocation because a secondary carbocation is more stable than the primary carbocation. There can be two types of rearrangements. Shift of an alkyl group is called a 1,2-alkyl shift. The most common carbocation rearrangements involve a carbocation rearranging into a more stable carbocation, such as 2o → 3o with resonance.
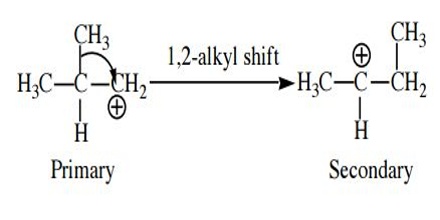
Rearrangements that transform a carbocation into another of apparently equal stability are less common, but they do occur. So before invoking this kind or rearrangement ask yourself if a better rearrangement, or some other mechanism step, is possible. Rearrangement to a less stable carbocation is very unusual, but also does occur. This is the pathway of last very last resort. All other reasonable options must be ruled out first.