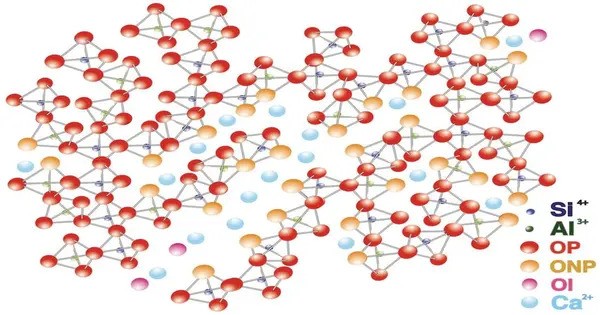
Calcium aluminoferrite (Ca2(Al,Fe)2O5) is a dark brown crystalline phase commonly found in cements. It is a compound found in the mineral composition of Portland cement. It is one of the four main clinker phases in Portland cement, along with tricalcium silicate (C3S), dicalcium silicate (C2S), and tricalcium aluminate (C3A). In the cement industry it is termed tetra-calcium aluminoferrite or ferrite.
Key Characteristics and Role
- Composition: It consists of calcium, aluminum, iron, and oxygen.
- Formation: C4AF forms during the clinkerization process at high temperatures, typically around 1,450–1,500°C, when limestone, clay, and iron-rich materials are heated together.
- Hydration: Upon mixing with water, calcium aluminoferrite undergoes hydration and reacts to form calcium aluminate and iron hydroxides. This phase is less reactive compared to others like C3A (tricalcium aluminate) and contributes to slower setting times.
- Function: It plays a role in the cement’s overall strength development and resistance to chemical attack, particularly sulfate resistance.
- Color: C4AF gives Portland cement its characteristic gray color, especially when combined with other compounds.
C4AF is often referred to as “the iron phase” due to its iron content and is known to contribute to the color and some of the properties of cement, particularly during hydration. In cement chemist notation (CCN), it is abbreviated as C4AF meaning 4CaO·Al2O3·Fe2O3 in the oxide notation. It also exists in nature as the rare mineral brownmillerite.
Occurrences
In Cement Production: Calcium aluminoferrite is primarily found in Portland cement clinker. The mineral forms during the high-temperature kiln process when raw materials like limestone, clay, and iron ore are heated together.
Cement Clinker: It forms alongside the other clinker minerals (tricalcium silicate, dicalcium silicate, and tricalcium aluminate) and is an essential phase in the final product.
Natural Occurrence: Although it is not widely found in nature in significant quantities, calcium aluminoferrite can form in certain metamorphic rocks or igneous rocks under high-temperature conditions where calcium, aluminum, and iron are present together. However, it is much more commonly synthesized in cement plants.
Calcium aluminoferrite is a complex mineral important in cement chemistry, contributing to the setting and hardening processes of Portland cement. It typically forms during the high-temperature clinker production process, and although it can occur naturally in specific geologic conditions, it is most commonly found in manufactured cement. Its chemical stability and low reactivity with water make it a minor but essential component in the cement industry.