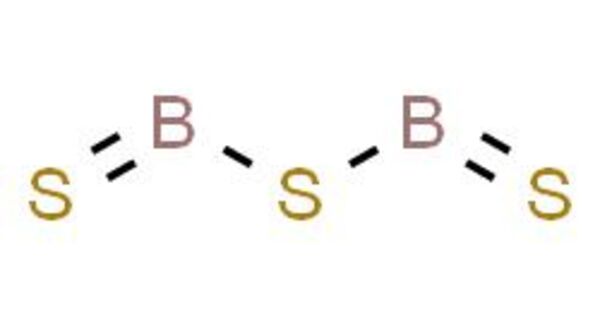
Boron sulfide is a chemical compound with the formula B2S 3. It’s a white, moisture-sensitive solid. It has a polymer structure. The material has sparked interest as a component of “high-tech” glasses and as a reagent for the synthesis of organosulfur compounds.
Boron sulfide interacts with water, yielding boric acid and hydrogen sulfide. It is also soluble in numerous organic solvents. It should be handled with caution due to its ability to emit poisonous gasses when decomposed at high temperatures.
Properties
Boron sulfide is a white solid that can also appear yellowish or brownish due to impurities. It is relatively stable in air but decomposes at high temperatures, releasing sulfur dioxide and leaving boron oxide behind.
- Chemical formula: B2S3
- Molar mass: 117.80 g/mol
- Appearance: colorless crystals
- Density: 1.55 g/cm3, solid
- Melting point: 563 °C (1,045 °F; 836 K)
- Boiling point: decomposes at high T
- Solubility in water: decomposes
- Solubility: soluble in ammonia
Reactions
Like the sulfides of silicon and phosphorus, B2S3 reacts with traces of water, including atmospheric moisture to release H2S. This hydrolysis is described by the following idealized equation:
B2S3 + 3 H2O → B2O3 + 3 H2S
B2S3 readily forms glasses when blended with other sulfides such as P4S10. Such glasses do not absorb mid-frequencies of Infra-red energy relative to conventional borosilicate glasses. Some of these ternary phases that are fast ion conductors.
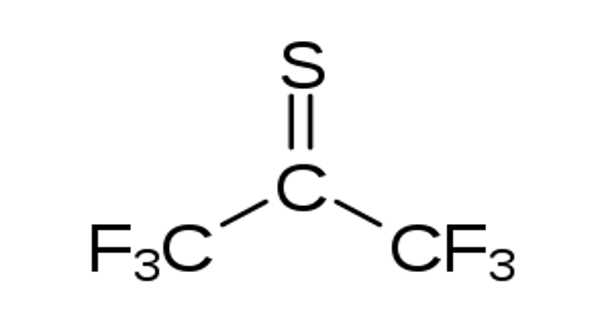
B2S3 converts ketones into the corresponding thiones. For example, the conversion of benzophenone to its thione proceeds as follows:
B2S3 + 3 (C6H5)2C=O → B2O3 + 3 (C6H5)2C=S
In practice, B2S3 would be used in excess.
Applications
Boron sulfide is primarily used in the synthesis of boron nitride and other boron compounds. It can also be employed as a precursor in the production of materials such as ceramics and glasses.