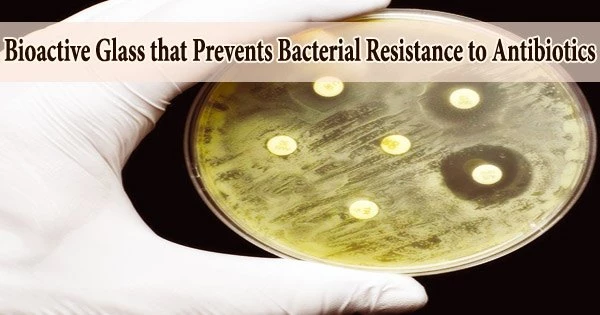
A straightforward procedure could significantly reduce infections connected to medical equipment like catheters, dental implants, orthopaedics, and wound dressings.
Bioactive glass, a substance utilized in numerous medical devices and clinical surfaces, has had its antimicrobial qualities greatly improved by researchers at Aston University.
The Aston University team has already created bioactive glass that kills germs and is infused with a single metal oxide, either copper, cobalt, or zinc. Using pairings of metal oxides in the material, their most recent research discovered that some combinations were more than 100 times more effective at killing bacteria than using single oxides alone.
The sort of bioactive glass being used in clinical settings frequently as a bone filler does not contain antimicrobial agents. Bioactive glass is created from highly pure chemicals intended to induce specific biological activity.
The Aston University study shown that mixing metal oxides can enhance the antibacterial capabilities of bioactive glass, and the researchers think this strategy could be used for other clinically relevant materials.
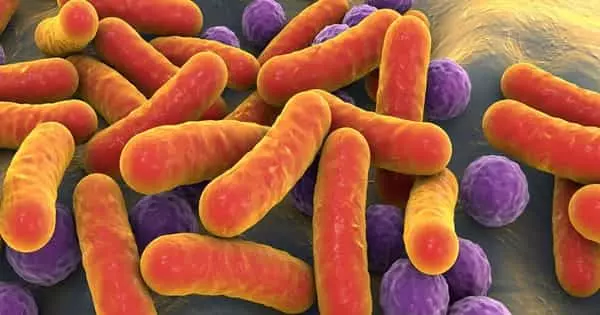
New methods to prevent infections are urgently needed since many bacteria that cause infections, like Escherichia coli and Staphylococcus aureus, are becoming more and more resistant to drugs.
We have shown that co-doping surfaces with these combined antimicrobial metals, including copper, zinc and cobalt, could reduce bacterial adhesion and colonisation to surfaces or devices used in clinical practice. The use of antimicrobial metals is potentially the way forward, given discovery of new antibiotics is currently limited. We would urge manufacturers to investigate whether our new approach could be used for their biomedical materials.
Dr. Tony Worthington
Professor Richard Martin, who led the research at Aston University’s Engineering for Health Research Group, said:
“Antibiotic drugs have been used in combination since the 1950s, as two antimicrobials can broaden the spectrum of coverage by aiming for different bacterial targets at the same time. Our research is the first to show that this combination approach can work with materials as well.”
In order to make bioactive glass, Professor Martin and his associates Drs. Tony Worthington and Farah Raja laced it with trace amounts of cobalt, copper, or zinc oxides as well as mixtures of two of the three oxides.
They sterilized the powder they created from these by grinding it, and then they added it to colonies of Candida abicans, E. coli, and S. aureus. They measured the bacterial and fungal death rates over a 24-hour period to evaluate the effects of conventional glass with glass with either single metal oxides or mixtures of metal oxides.
Both individually and collectively, the metal oxide-laced glass outperformed the glass. The most effective combination against the bacteria was copper with either cobalt or zinc, followed by cobalt and zinc.
While copper and zinc were equally efficient against S. aureus, both copper combinations were over one hundred times more potent than single oxides at killing E. coli. The combination of cobalt and zinc had the most impact on the fungus.
Professor Martin said: “It was exciting to run our experiments and find something that is significantly better at stopping infection in its tracks and could potentially reduce the number of antibiotic treatments that are prescribed. We believe combining antimicrobial metal oxides has significant potential for numerous applications including implant materials, hospital surfaces and wound healing dressings.”
Dr. Worthington added: “We have shown that co-doping surfaces with these combined antimicrobial metals, including copper, zinc and cobalt, could reduce bacterial adhesion and colonisation to surfaces or devices used in clinical practice. The use of antimicrobial metals is potentially the way forward, given discovery of new antibiotics is currently limited. We would urge manufacturers to investigate whether our new approach could be used for their biomedical materials.”