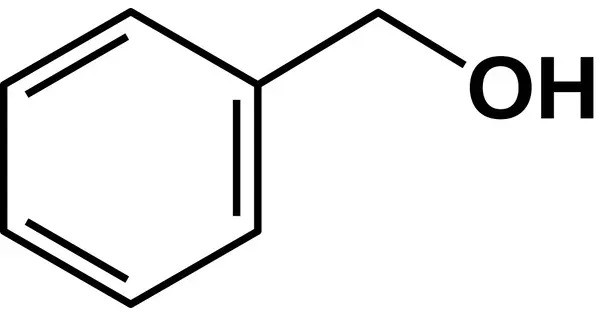
Benzyl alcohol (also known as α-cresol) is an aromatic alcohol with the formula C6H5CH2OH. It is a colorless liquid with a mild pleasant odor, and it is commonly used as a solvent, preservative, and in the production of other chemicals. It is useful as a solvent for its polarity, low toxicity, and low vapor pressure. It is soluble in water (about 4% by weight) and mixes well with many organic solvents.
Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide.
Properties
- Chemical formula: C7H8O
- Molar mass: 108.140 g·mol−1
- Appearance: Colorless liquid
- Odor: Slightly aromatic
- Density: 1.044 g/cm3
- Melting point: −15.2 °C (4.6 °F; 257.9 K)
- Boiling point: 205.3 °C (401.5 °F; 478.4 K)
- Solubility in water: 3.50 g/100 mL (20 °C), 4.29 g/100 mL (25 °C)
- Solubility in other solvents: Miscible with benzene, methanol, chloroform, ethanol, ether, acetone
Natural occurrences
Benzyl alcohol is produced naturally by many plants and is commonly found in fruits and teas. It is also found in a variety of essential oils including jasmine, hyacinth and ylang-ylang. It is also found in castoreum from the castor sacs of beavers. Benzyl esters also occur naturally.
Preparation
Benzyl alcohol is produced industrially from toluene via benzyl chloride, which is hydrolyzed:
C6H5CH2Cl + H2O → C6H5CH2OH + HCl
Another route entails hydrogenation of benzaldehyde, a by-product of the oxidation of toluene to benzoic acid.
For laboratory use, Grignard reaction of phenylmagnesium bromide (C6H5MgBr) with formaldehyde and the Cannizzaro reaction of benzaldehyde also give benzyl alcohol. The latter also gives benzoic acid, an example of an organic disproportionation reaction.
Reactions
Like most alcohols, it reacts with carboxylic acids to form esters. In organic synthesis, benzyl esters are popular protecting groups because they can be removed by mild hydrogenolysis.
Benzyl alcohol reacts with acrylonitrile to give N-benzylacrylamide. This is an example of a Ritter reaction:
C6H5CH2OH + NCCHCH2 → C6H5CH2N(H)C(O)CHCH2
Applications
Benzyl alcohol is used as a general solvent for inks, waxes, shellacs, paints, lacquers, and epoxy resin coatings. Thus it can be used in paint strippers, especially when combined with compatible viscosity enhancers to encourage the mixture to cling to painted surfaces.
- Solvent: It is often used as a solvent in various industries, including in paints, lacquers, and inks.
- Preservative (Antimicrobial Agent): It is used in pharmaceutical and cosmetic products to preserve the integrity of formulations by inhibiting microbial growth.
- Fragrance and Flavoring: Due to its pleasant smell, it is used in perfumes and as a flavoring agent in some food products.
- Antiseptic: It has mild antiseptic properties and is sometimes used in minor medical applications.
Safety Considerations
- It is considered relatively safe for external use but can cause irritation to the skin, eyes, or respiratory system if exposed in large amounts.
- Toxicity: Ingesting large quantities may lead to symptoms of toxicity such as headaches, nausea, and dizziness.