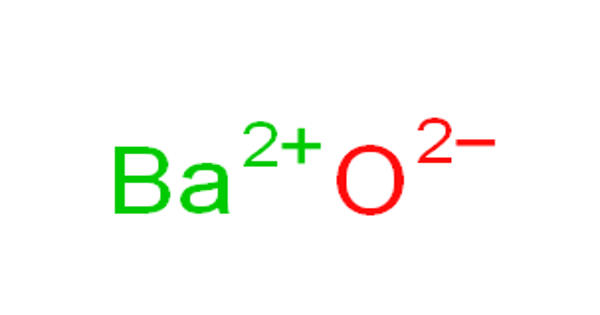Barium oxide is a white hygroscopic non-flammable compound. It appears as a white to yellow powder. It is also called Barium monoxide or Barium protoxide or Calcined baryta. It has a cubic structure and is used in cathode ray tubes, crown glass, and catalysts. It has the chemical formula BaO and it is also hygroscopic, which means it readily absorbs moisture from the air. It is harmful to human skin and if swallowed in large quantity causes irritation. Excessive quantities of barium oxide may lead to death.
Preparation
Barium oxide is made by heating barium carbonate. It may also be prepared by thermal decomposition of barium nitrate. Likewise, it is often formed through the decomposition of other barium salts.
2Ba + O2 → 2BaO
BaCO3 → BaO + CO2
It is prepared by heating barium carbonate with coke, carbon black or tar or by thermal decomposition of barium nitrate.

Properties
Barium protoxide is a cubic structure that appears as a white to yellow colored powder which is non-flammable. The exact mass and the monoisotopic mass of Barium monoxide is 153.9 g/mol. The number of hydrogen bond acceptors equals one and the number of hydrogen bond donors equals zero.
- Compound Formula: BaO
- Molecular Weight: 153.326
- Appearance: White to very pale yellow crystalline solid
- Melting Point: 1,923° C (3,493° F)
- Boiling Point: ~2000 °C (3,632° F)
- Density: 5720 kg/m-3
- Solubility in H2O: N/A
- Exact Mass: 153.9 g/mo
- Monoisotopic Mass: 153.900157 Da
Uses
- Barium oxide is used as a coating for hot cathodes, for example, those in cathode ray tubes.
- It is widely used as a drying agent for solvents, in cathode ray tubes, catalysts, and crown glass.
- Barium oxide also has use as an ethoxylation catalyst in the reaction of ethylene oxide and alcohols, which takes place between 150 and 200 °C.
- It is used as a drying agent for gasoline and solvents.
- It is also a source of pure oxygen through heat fluctuation.
- It is used in the process of isomer separation.
- It is used as an excellent oxidizing.
- It is used as a strong reducing agents.
Health hazards
It is a toxic and corrosive compound which is non-combustible and water sensitive. Inhalation, swallowing or contact (with skin or eyes) with vapours, substance or dust causes severe burns or death. On reacting with water it generates heat that increases the fumes concentration in the air. When this compound catches fire it liberates irritating, toxic, and corrosive gases.
Information Source: