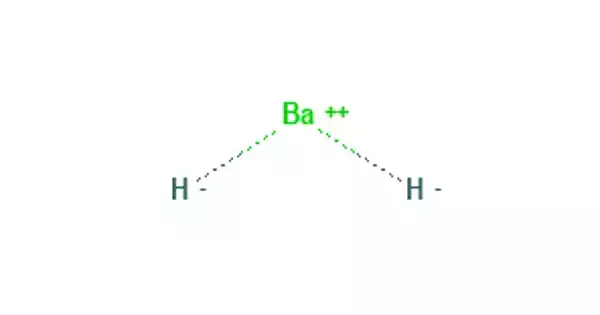
Barium hydride (BaH2) is a chemical compound with the formula BaH2. When mixed with water, it emits hydrogen gas. It’s used as a hydrogen source in experimental rockets and fuel cells.
Barium is a chemical element with the atomic number 56 and the symbol Ba. It is a soft, silvery alkaline earth metal and the fifth element in Group 2. Because of its high chemical reactivity, barium is never found as a free element in nature. A hydride is the anion of hydrogen, H−, in chemistry. The term is used in a broad sense. At one extreme, all compounds containing covalently bound H atoms are referred to as hydrides: water (H2O) is an oxygen hydride, ammonia is a nitrogen hydride, and so on.
Properties
It appears as a solid and also as a molecular polymer (upto decamer). It does not have any common uses. All beryllium compounds are quite toxic. It is a simple lightweight metal hydride well known for its hydrogen storage capability.
- Molecular Weight: 139.34288
- Appearance: solid
- Melting Point: 675 °C (1,247 °F; 948 K) decomposes
- Boiling Point: N/A
- Density: 4.21 g/cm3
- Solubility in H2O: N/A
- Color: white to gray crystal
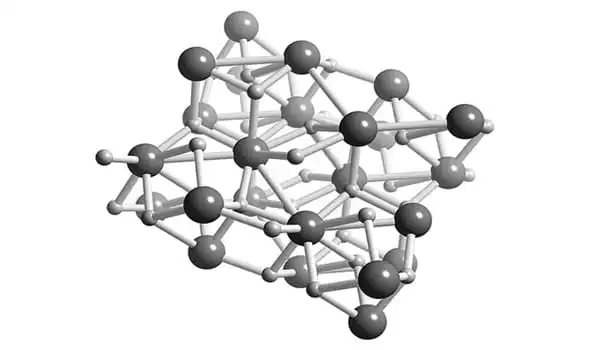
Structure
BeH2 is usually formed as an amorphous white solid, but a hexagonal crystalline form with a higher density (~0.78 g cm-3) was reported, prepared by heating amorphous BeH2 under pressure, with 0.5-2.5% LiH as a catalyst.
Preparation
Barium hydride can be prepared by dissolving elemental barium (Ba) with hydrogen at temperatures between 150-200 °C:
Ba + H2 → BaH2
When mixed with water, it emits hydrogen gas. It’s used as a hydrogen source in experimental rockets and fuel cells. It is created by pyrolyzing di-tert-butylberyllium or treating an ethereal solution of beryllium borohydride with triphenylphosphine.
Reactions
When barium hydride reacts with oxygen and water, it forms barium hydride. When combined with a solid oxidant such as a halide or chromate, it becomes explosive. It is insoluble in organic solvents, but at 160°C, it reacts with tertiary amines to form stable adducts, such as (R3N• BeH2)2. Beryllium hydride was once considered as a rocket fuel and a nuclear reactor moderator. Toxicity has been a significant impediment to commercialization.