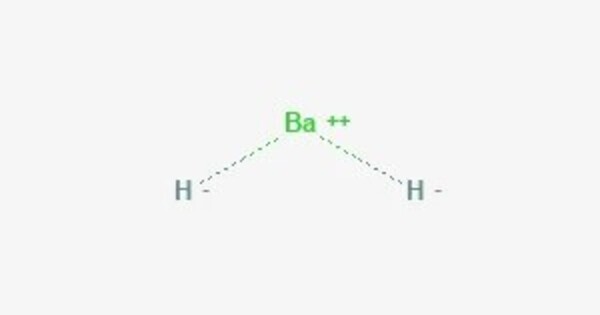
Barium hydride is a chemical compound with the chemical formula BaH2. It appears as a white or colorless crystalline solid. It is not very soluble in water, but reacts with water to form barium hydroxide (Ba(OH)₂) and hydrogen gas (H₂). It is a strong reducing agent and can react with moisture in the air, making it quite reactive and prone to hydrolysis. It has a crystalline structure where barium is in a metal lattice with hydride ions.
It does not naturally occur in significant quantities in the environment. Instead, it is primarily used in industrial applications and research settings.
Preparation
Barium hydride can be prepared by reacting elemental barium with hydrogen at relatively high temperatures between 150-200 °C:
Ba + H2 → BaH2
Barium hydride is not a common substance found in nature. It is typically synthesized in laboratory settings. Some of the methods used to prepare barium hydride include:
- Direct Synthesis: Reacting barium with hydrogen gas at high temperatures.
- Hydride Exchange: Converting barium salts with a hydride.
Reactions
Barium hydride reacts with oxygen and water. It is easily explosive when it is mixed with a solid oxidant such as a halide or chromate.
Properties
- Chemical formula: BaH2
- Molar mass: 139.343 g/mol
- Appearance: white to gray crystals
- Density: 4.16 g/cm3
- Melting point: 675 °C (1,247 °F; 948 K) decomposes
- Solubility in water: Reaction
- Boiling Point: it decomposes rather than boiling; its thermal decomposition starts around 600°C (1112°F).
Uses
Barium hydride is used in some chemical reactions and can serve as a reducing agent. It’s also used in some specialized industrial processes.
Safety
It should be handled with care due to its reactivity with water and potential toxicity. It is important to follow proper safety procedures when working with this compound.