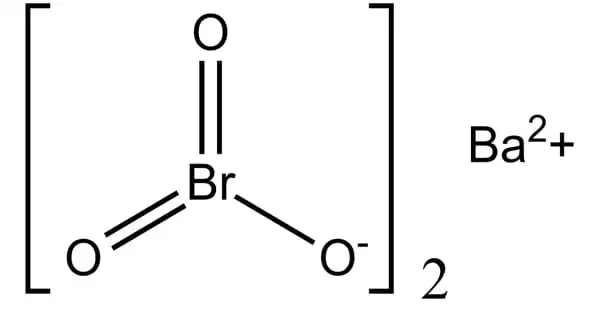
Barium bromate, with the chemical formula Ba(BrO3)2, is a chemical compound comprised of the barium ion and the bromate ion. It has the appearance of a white crystalline solid or powder. It has a slightly higher density than water and is marginally soluble in it. It is employed as a corrosion inhibitor and in the production of other compounds.
Although barium bromide is a metallic alkaline in nature, it is never seen in pure form. This is due to the fact that barium bromide interacts quickly with air. Barium bromide also interacts with oxygen, sulfur, and carbon to generate barium compounds.
Properties
The molecular mass of barium bromide is 297.14 gram per mole. The melting point is high. The inorganic compound melts at 857-degree Celsius. The boiling point is 1835-degree Celsius. The density is 4.78 g/cm3. It is an inorganic compound. This chemical compound is also called barium bromide anhydrous or barium (2+) dibromide.
- Molecular Weight: 393.13
- Appearance: White powder
- Melting Point: N/A
- Boiling Point: 18350C
- Density: 3.99 g/cm3
- Solubility in H2O: N/A

Preparation
Barium bromate can be prepared by reacting potassium bromate with barium chloride:
2 KBrO3 + BaCl2 → Ba(BrO3)2 + 2 KCl
The barium bromide solutions react with the salts of sulfate and produce a solid precipitate of barium sulfate:
BaBr2 + SO42- → BaSO4 + 2Br–
Barium bromide reacts with oxalic acid to produce solid precipitates of barium oxalate:
BaBr2 + C2O42- → BaC2O4 + 2Br–
It is a white odourless powder.
The reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride:
BaBr2 + F– → BaF2 + 2Br–
BaF is a colorless solid that occurs in nature.
The barium bromide formula also refers to the barium dibromide formula, which functions as a salt when dissolved in water. Barium bromide salt crystallizes in the lead chloride theme, resulting in deliquescent orthorhombic white crystals.
Toxicity
It is extremely poisonous when ingested or inhaled. When in touch with organic materials, it can catch fire and explode if heated above 300°F.
There are various documented risks associated with the use of barium bromide. The inorganic substance barium can obstruct the entrance of intracellular potassium. As a result, potassium is transferred from the extracellular compartment to the intracellular compartment. There is a decrease in the resting membrane potential, which electrically unexcites the muscle fibers. As a result, it has the potential to cause paralysis.
Due to the presence of barium, an alkaline metal, barium bromide is an alkaline metal. It’s preferable to take precautions. Only consume a tiny amount of water-soluble barium bromide or other barium compounds.