Azelaic Acid
Definition
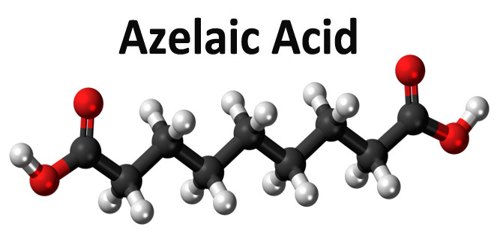
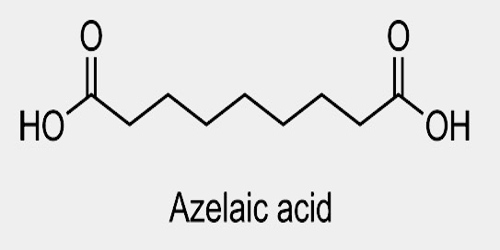
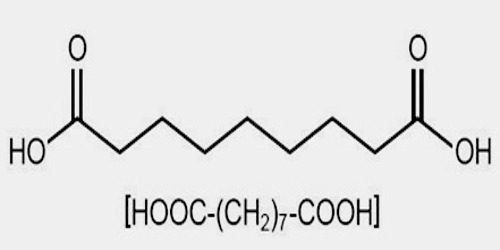
Azelaic acid is a naturally occurring acid, which is an organic compound with the formula HOOC(CH2)7COOH. It is found in wheat, rye, and barley. It helps the skin to renew itself more quickly and therefore reduces pimple and blackhead formation. It also helps to kill the bacteria that cause acne and rosacea.
In plants, azelaic acid serves as a “distress flare” involved in defense responses after infection. It serves as a signal that induces the accumulation of salicylic acid, an important component of a plant’s defensive response.
Azelaic acid exerts its keratolytic and comedolytic effects by reducing the thickness of the stratum corneum and decreasing the number of keratohyalin granules by reducing the amount and distribution of filaggrin in epidermal layers. Azelaic acid also possesses a direct anti-inflammatory effect due to its scavenger activity of free oxygen radical. This drug is used topically to reduce inflammation associated with acne and rosacea.
Production and Occurrences of Azelaic Acid
The known processes for the production of azelaic acid are based upon the treatment of natural products, from which the acid is obtainable in more or less good yields by saponification and oxidation of the hydrolysis products. Oleic acid and ricinoleic acid yield azelaic acid by ozonization and decomposition of the ozonides. Ricinoleic acid can also be oxidized to azelaic acid by nitric acid. In view of the fact that natural oils are the basis of all of these processes and are mixtures of higher fatty acid esters,-it can be seen that it is difficult to obtain a pure azelaic acid product in this way.
Azelaic acid is industrially produced by the ozonolysis of oleic acid. The side product is nonanoic acid. It is produced naturally by Malassezia furfur, which is also known as Pityrosporum ovale, yeast that lives on normal skin. The bacterial degradation of nonanoic acid gives azelaic acid.
Process for the production of azelaic acid which comprises condensing methoxy-butene-ine- with a member selected from the group consisting of -chloro -methoxy-pentene-chloro-n1ethoxy-pentenel and mixtures thereof in an alcoholic solution of an alkali metal hydroxide in the presence of a cuprous compound thereby forming, dimethoxy-nonadiene, hydrogenating the latter thereby producing, dimethoxynonane and oxidizing the latter with nitric acid.
Azelaic acid (AZA) is a naturally occurring saturated nine-carbon dicarboxylic acid (COOH (CH2)7-COOH). It possesses a variety of biological actions both in vitro and in vivo. Interest in the biological activity of AZA arose originally out of studies of skin surface lipids and the pathogenesis of hypochromia in pityriasis versicolor infection. Later, it was shown that Pityrosporum can oxidize unsaturated fatty acids to C8-C12 dicarboxylic acids that are cornpetitive inhibitors of tyrosinase in vitro.
Uses and Effects of Azelaic Acid
Azelaic Acid is naturally antibacterial which makes it a great treatment for acne. When applied topically, it reduces the growth of bacteria in a follicle, helps to reduce inflammation and remove dead skin cells to prevent future acne breakouts.

In lubricant industries it is used as a thickening agent in lithium complex grease. With hexamethylenediamine, azelaic acid forms Nylon-6,9, which finds specialized uses as a plastic.
Azelaic Acid can be used on just about any skin type, including sensitive skin, as it has the ability to reduce inflammation. Anyone with rosacea, especially those with acne rosacea, will find it makes an excellent treatment for calming and soothing their inflamed skin.
Azelaic acid topical side effects:
- severe burning, stinging, or warmth;
- severe itching or tingling;
- severe redness, dryness, peeling, or other irritation; or
- Changes in skin color.
Azelaic acid is less expensive than certain other prescription acne preparations, but it is much more expensive than nonprescription benzoyl peroxide preparations. Whether it is safe and effective when used in combination with other agents is not known.
Reference: