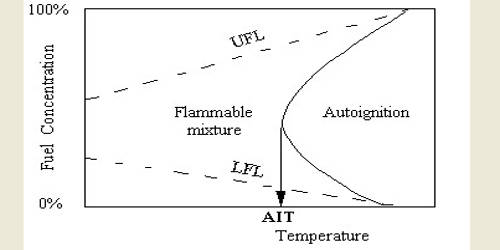
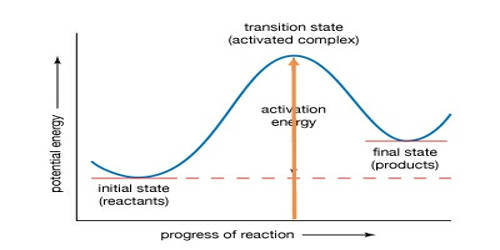
Autoignition refers to a homogeneous ignition of the fuel-air mixture. The autoignition temperature or kindling point of a substance is the lowest temperature at which it spontaneously ignites in a normal atmosphere without an external source of ignition, such as a flame or spark. Autoignition occurs when a mixture of gases or vapors ignites spontaneously with no external ignition source and after reaching a certain temperature, the autoignition temperature. This temperature is required to supply the activation energy needed for combustion. The temperature at which a chemical ignites decreases as the pressure or oxygen concentration increases. It is usually applied to a combustible fuel mixture. The autoignition temperature of a fluid varies considerably with circumstances.
- The ignition temperature of a substance is the least temperature at which the substance starts combustion. Flash Point is the lowest temperature that the vapors of material will ignite when exposed to an ignition source.
Substances that spontaneously ignite in a normal atmosphere at naturally ambient temperatures are termed pyrophoric. - Autoignition temperatures of liquid chemicals are typically measured using a 500-milliliter (18 imp fl oz; 17 US fl oz) flask placed in a temperature-controlled oven in accordance with the procedure described in ASTM E659. Flammable, volatile products are often tested for their Flash Point or Autoignition point to characterize them.
- The autoignition temperature of a mixture of gases or vapors is affected by pressure, vessel shape and volume, surface activity, contaminants, flow rate, reaction rate, droplet and mist formation, gravity, and reactant concentration.
The autoignition temperature is not an intrinsic property of the gases or vapors but is the lowest temperature in a system where the rate of heat evolved from the gases or vapors increases beyond the rate of heat loss to the surroundings, resulting in the ignition. When measured for plastics, autoignition temperature can be also measured under elevated pressure and at 100% oxygen concentration. The test involves heating a vessel containing the liquid sample in an enclosed oven, then measuring when ignition occurs. Due to the hazardous nature of the test, this is all automatic. The resulting value is used as a predictor of viability for high-oxygen service. The main testing standard for this is ASTM G72.