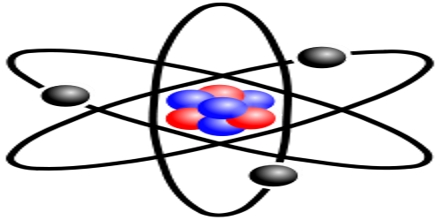
General objective of this lecture is to describe on Atoms and Molecules, in terms of Rutherford Model and Avogadro Constant. The “Rutherford Model” of the atom is a simple way of thinking of an atom. In this Model, electrons move around (orbit) the nucleus like planets moving around the sun. Electrons have a certain probability of being in a certain place at any time. This probability has a shape called an “orbital”. This lecture also focus on Avogadro Constant and how to use a mole.
Atoms and Molecules