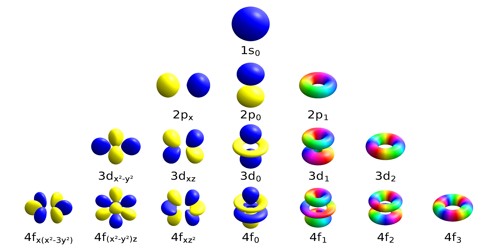
Atomic orbitals are the places surrounding the nucleus of an atom where the electrons are most likely to be at any given time. They are the quantum states of the individual electrons in the electron cloud around a single atom. It is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. Atomic orbitals are commonly designated by a combination of numerals and letters that represent specific properties of the electrons associated with the orbitals—for example, 1s, 2p, 3d, 4f.
The word ‘orbital’ is used because it was thought that electrons behaved similarly to the solar system, where the nucleus is like the sun and the electrons orbit like the planets. An orbital is the quantum mechanical refinement of Bohr’s orbit.
Specifically, atomic orbitals are the quantum states of the individual electrons in the electron cloud around a single atom. An orbital often is depicted as a three-dimensional region within which there is a 95 percent probability of finding the electron. The number of atomic orbitals in an element is defined by the period the element is in. Electrons move between orbitals depending on how fast they are moving and how many other electrons there are. Each orbital can fit two electrons and different orbitals have different shapes. The higher the probability of finding an electron at a given position, the larger the electron density at that position.
Atomic orbitals are regions of space in which electrons can be found. In atomic theory and quantum mechanics, an atomic orbital is a quantum number. Each such orbital can be occupied by one or two electrons. It is not possible to determine the exact location of an electron in an atom. The way orbitals are arranged is related to the electron configurations of atoms. However, the probability of finding an electron at a given position can be calculated. They were derived from descriptions provided by early spectroscopists of certain alkali metal spectroscopic lines as being sharp, principal, diffuse, and fundamental.