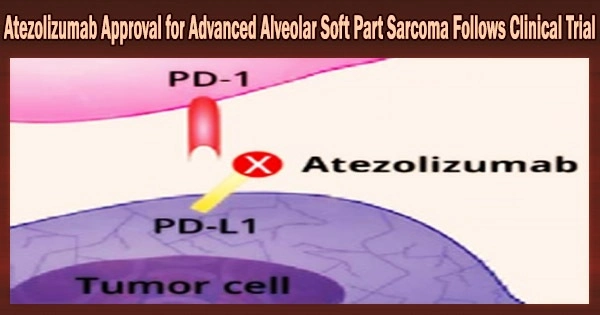
Atezolizumab, a monoclonal antibody treatment, for advanced Alveolar Soft Part Sarcoma (ASPS), a rare type of cancer. This means that Atezolizumab can now be used as a treatment option for patients with advanced ASPS who have received prior chemotherapy.
A clinical trial led by the National Cancer Institute (NCI), part of the National Institutes of Health, has resulted in the first approval of a treatment for advanced alveolar soft part sarcoma (ASPS).
The U.S. Food and Drug Administration (FDA) recently authorized the immunotherapy medication atezolizumab (Tecentriq) for the treatment of adults and children 2 years of age and older with ASPS that has expanded to other parts of the body or cannot be removed surgically.
ASPS is an extremely rare cancer that affects mostly adolescents and young adults. The approval was based on data from a non-randomized phase 2 trial (NCT03141684) funded by NCI and led by Dr. Alice Chen, M.D., of the Developmental Therapeutics Clinic in NCI’s Division of Cancer Treatment and Diagnosis (DCTD).
Atezolizumab was given to NCI under a joint research and development arrangement by Genentech, a company that is a part of the Roche Group and the maker of the medication. The study’s findings are currently being readied for publication.
“Forty percent of the patients were treated at the NIH Clinical Center in Bethesda,” said James H. Doroshow, M.D., director of DCTD. “Our ability to bring patients in from all over the world was a key factor in the ability to do the study.”
“This approval will make a huge impact in terms of a rare disease that has been particularly challenging to treat,” Dr. Chen noted.
This is the largest study on ASPS. It is moreover the first study carried out under the Experimental Therapeutics Clinical Trials Network, supported by the NCI, to lead to a medication approval. Sarcoma specialists from academic medical centers in North America were able to sign up patients for the research because to the network.
“This is a major milestone for investigators in the Experimental Therapeutics Clinical Trials Network, as well as for the ASPS patient community, and for research on rare cancers,” said Elad Sharon, M.D., of DCTD, who is one of the study leaders.
This study is an important example of collaboration between pediatric and medical oncology, allowing children with very rare cancers access to effective new therapies. The entire study team is grateful to the patients who participated in the study and made this work possible.
John W. Glod
This is also the first time atezolizumab has been approved for children. Dr. Chen noted that this was enabled by the participation of the Pediatric Oncology Branch in NCI’s Center for Cancer Research, which helped enroll children in the trial.
“This study is an important example of collaboration between pediatric and medical oncology, allowing children with very rare cancers access to effective new therapies,” said John W. Glod, M.D., Ph.D., of the Pediatric Oncology Branch. “The entire study team is grateful to the patients who participated in the study and made this work possible.”
About 80 people in the United States are diagnosed with ASPS every year. The soft tissue that surrounds and links the organs and other tissues is where the sickness often starts. Despite the disease’s gradual growth, once it has taken hold, it is frequently fatal, and chemotherapy has no effect.
About 50% of patients with metastatic disease are still alive after five years. Tyrosine kinase inhibitors are a class of new targeted medications that do not have long-lasting efficacy. Recently, however, immunotherapy drugs have shown promise as possible therapies for ASPS.
Anti-PD-L1 immune checkpoint inhibitor atezolizumab works by enhancing the immune system’s capacity to combat cancer. Atezolizumab has been licensed by the FDA for the treatment of individuals with a variety of cancers, including lung, melanoma, and liver cancer.
Atezolizumab was given the breakthrough treatment designation by the FDA in 2020 to treat patients with metastatic or unresectable ASPS. Atezolizumab, a medication used to treat a critical disease, was given this designation because it satisfied FDA requirements for a fast track to development and approval.
Atezolizumab was designated an orphan medication for soft tissue sarcoma later that year by the FDA. Companies are encouraged to create a medicine for uncommon diseases by this status.
The phase 2 trial enrolled 49 ethnically diverse patients ages 2 and older with metastatic ASPS, who were given an infusion of atezolizumab every 21 days. According to their doctor’s evaluation, about a third of the patients responded to the treatment with some degree of tumor shrinking. Most of the other patients experienced stable disease.
Patients were offered the option to quit treatment after two years and take a break from it for up to two years while being closely monitored. No patient who stopped receiving treatment experienced illness progression during that time.
Serious side effects occurred in 41% of patients receiving atezolizumab; these included anemia, diarrhea, rash, dizziness, hyperglycemia, and pain in the extremities. However, no patients came off the study because of side effects.
“This approval represents a victory for rare diseases, which are understudied in clinical trials,” said Dr. Chen. “For this approval to go through in a rare disease, and to be able to make an impact on these young people’s lives, is very significant.”
Atezolizumab is currently the subject of additional studies for people with ASPS, including administration of the medication in combination with other treatments.