Some nucleic acids, such as messenger RNA (mRNA), DNA, and related molecules, have been shown in laboratory settings to be effective disease treatments, raising hopes that improved human therapies for conditions such as cancer and genetic and vascular diseases will be developed. Therapeutic nucleic acids (TNA) have the potential to be used as tools, drugs, or vaccines for the treatment, prevention, or diagnosis of genetic conditions.
“At the moment, a handful of TNAs have been approved for clinical applications, and clinical trials are in the works,” said Dr. Changyi Johnny Chen, a professor of surgery and molecular and cellular biology at Baylor College of Medicine. “However, progress is hampered primarily because TNAs are notoriously difficult to deliver effectively in living organisms.”
When naked nucleic acids enter the body, they face a number of challenges that limit their availability. For example, they may encounter endothelial barriers that limit their desired distribution. They may also have to deal with rapid enzymatic digestion, liver retention, renal clearance, and unexpected accumulation in the ‘wrong’ tissue. TNAs have the potential to activate the immune system and cause severe side effects.
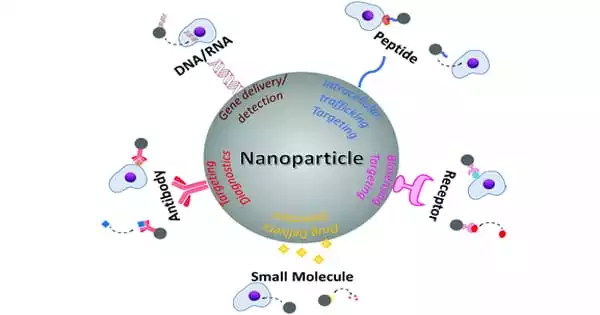
Antibodies help therapeutic nucleic acids get into nanoparticles more effectively. This improved technological platform can be used to develop new treatments, molecular diagnosis strategies, or vaccines for various diseases.
Dr. Changyi Johnny Chen
“TNAs are promising approaches to treating a variety of conditions, despite the obstacles that limit their applications. My lab is interested in developing strategies to improve their safety and efficacy through improved delivery “Chen stated.
Liposomes, which are artificially formed tiny spherical sacs of lipids enclosing a water droplet that carries TNAs, are one of the most widely used delivery systems. Virus-based delivery systems have also been employed. Both strategies have been beneficial, but they pose efficacy and safety concerns.
LGA-PEI nanoparticles
Dr. Qizhi Cathy Yao’s lab, professor of surgery, molecular virology and microbiology, pathology and immunology, and Chen’s lab, have been working on developing effective pancreatic cancer therapeutics since they moved to Baylor in 2002.
“Previous research has shown that certain RNAs, such as microRNA-198 (miR-198), a natural tumor suppressor, may have clinical applications in cancer,” Chen explained. “If they did, we’d need an efficient and secure delivery system, which wasn’t available at the time.”
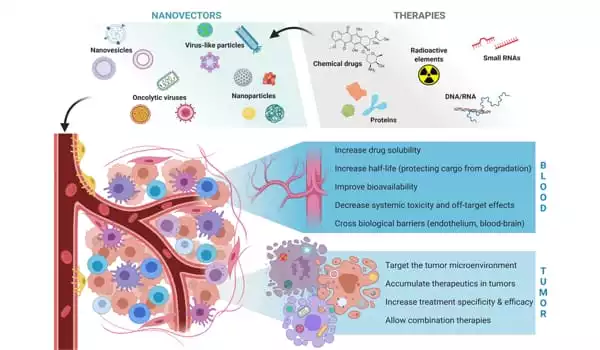
“My laboratory has been developing TNA delivery systems for many years,” Chen explained. “Finally, we designed, synthesized, and characterized a new approach called LGA-PEI, which is based on polyethyleneimine (PEI) polymers modified with lactic-co-glycolic acid (LGA).”
LGA-PEI polymers spontaneously form nanoparticles with layers that protect TNAs like miR-198 from enzymatic digestion and improve cellular uptake. In the case of cancer, increased uptake of TNA-loaded nanoparticles could result in improved anti-cancer effects. Experiments with cells in the lab and mouse models revealed that TNA delivery via LGA-PEI nanoparticles was less toxic than previous nanoparticles.
“Our results were encouraging,” Chen said, “but we still wanted to improve our delivery system even more.” “We added antibodies or antibody fragments to our LGA-PEI nanoparticles in this study to fine-tune their delivery to specific cancers.”
Antibodies improve delivery
Antibodies are proteins produced by the immune system that recognize and bind to specific cell surface markers. When antibodies are attached to other molecules or nanoparticles, they can mediate their delivery to specific cancer cells that display unique markers that are not found in other cells, allowing them to target cancer cells while sparing normal cells.
Chen and colleagues, for example, previously demonstrated that the protein mesothelin (MSLN) is abundantly expressed in some human cancers, including pancreatic cancer. MSLN, on the other hand, has limited expression in normal tissues of the body, making it a potential target for cancer therapy.
“In this study, we successfully linked a fragment antibody against MSLN to our LGA-PEI polymer and used fluorescent tags to demonstrate improved MSLN-targeted delivery of TNAs in pancreatic cancer cells grown in the lab and in animal models,” Chen explained.
The Chen lab is interested in conducting studies to compare the effectiveness of this active-targeting delivery system in inhibiting tumor growth and metastasis, as well as improving overall survival of tumor-bearing animals, to passive-targeting delivery or other non-targeting delivery systems.
The improved nanoparticle delivery system is a technological platform that can be applied to other types of cancer or diseases to develop new treatments, molecular diagnosis strategies, or vaccines. The full study can be found in the journal Pharmaceuticals.