Researchers discovered a crucial relationship between the distribution of antibiotic-resistance genes and the emergence of resistance to new treatments in certain organisms. Bacteria exposed to higher quantities of antibiotics frequently carry several identical copies of protective antibiotic resistance genes, which are connected to ‘jumping genes’ that can migrate between strains. Duplicate genes give a method for resistance to propagate and the ability to evolve resistance to new treatments.
Duke University biomedical engineers discovered a critical relationship between the distribution of antibiotic-resistance genes and the emergence of resistance to new treatments in certain organisms.
According to the study, bacteria exposed to greater amounts of antibiotics frequently have several identical copies of protective antibiotic resistance genes. These duplicated resistance genes are frequently connected to “jumping genes” called transposons, which can migrate from strain to strain. Not only does this provide a method for resistance to propagate, but having several copies of a resistance gene can also serve as a trigger for evolution to produce resistance to new types of medications.
The results were published in the journal Nature Communications.
Bacteria are constantly evolving under many pressures, and elevated duplication of certain genes is like a fingerprint left at the crime scene that allows us to see what kinds of functions are evolving very quickly.
Rohan Maddamsetti
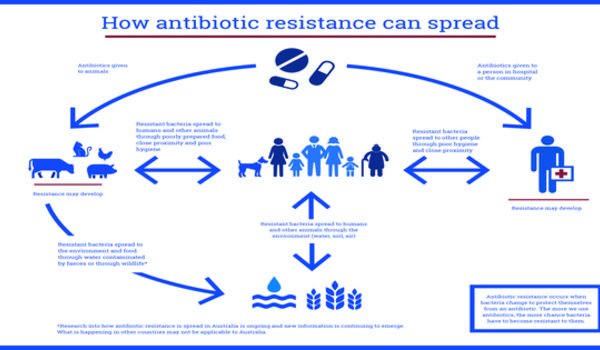
Earlier research by the Lingchong You lab revealed that 25% of bacterial pathogens can spread antibiotic resistance through horizontal gene transfer. They also demonstrated that the presence of antibiotics does not accelerate the rate of horizontal gene transfer, implying that something else is causing the genes to spread.
“Bacteria are constantly evolving under many pressures, and elevated duplication of certain genes is like a fingerprint left at the crime scene that allows us to see what kinds of functions are evolving very quickly,” said Rohan Maddamsetti, a postdoctoral fellow working in the laboratory of Lingchong You, the James L. Meriam Distinguished Professor of Biomedical Engineering at Duke.
“We hypothesized that bacteria under attack from antibiotics would often have multiple copies of protective resistance genes, but until recently we didn’t have the technology to find the smoking gun.”
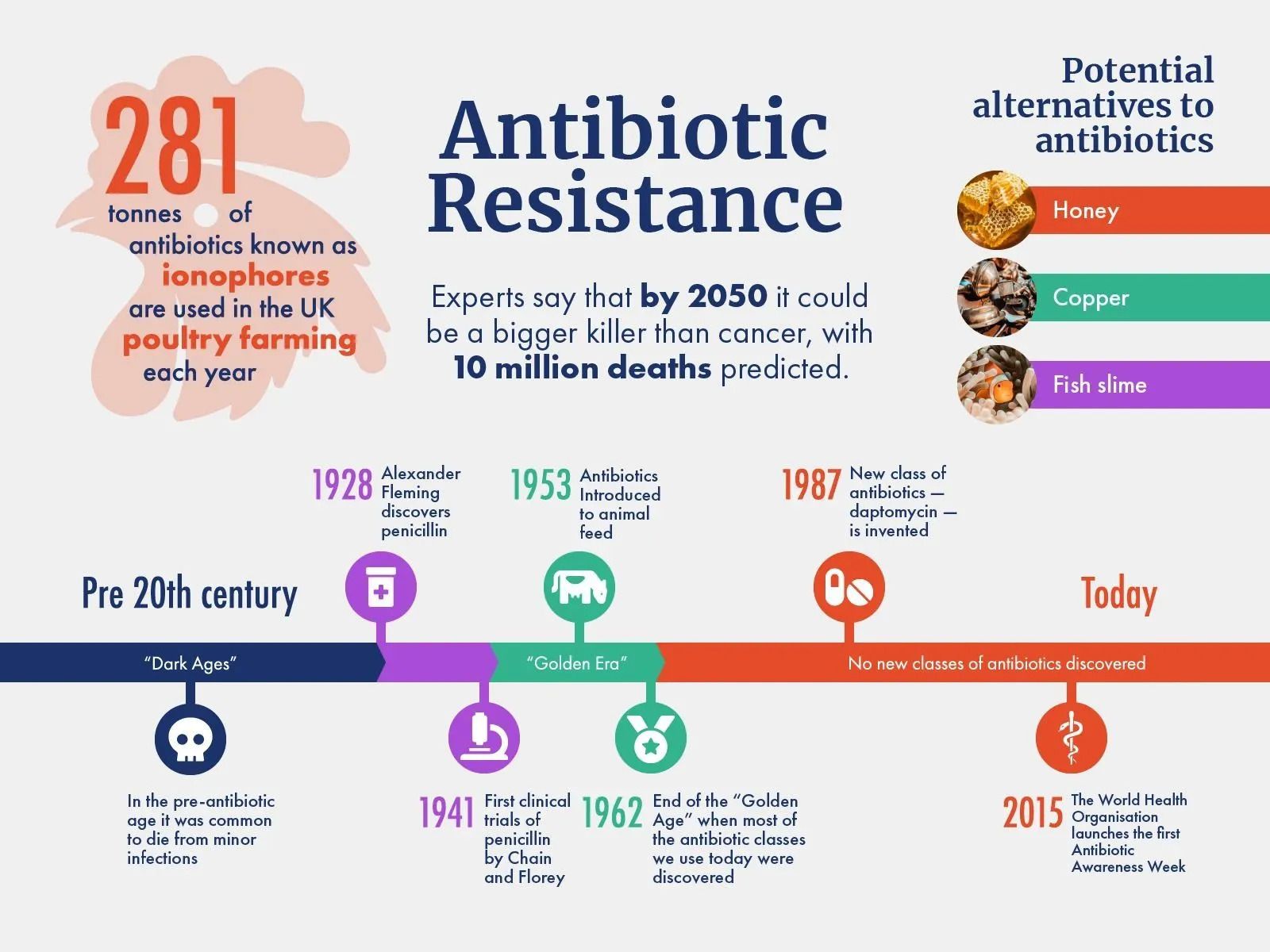
Traditional DNA-reading technology copies short snippets of genes and counts them up, making it hard to determine whether high counts of specific sequences are actually in the sample or if they are being artificially amplified by the reading process. In the past five years, however, complete genome sequencing with long-read technology has become more common, allowing researchers to spot high levels of genetic repetition.
Maddamsetti and coauthors counted the number of resistance gene repetitions in bacterial pathogen samples collected from various habitats. They revealed that those living in areas with higher levels of antibiotic usage — humans and cattle — have more identical copies of antibiotic resistance genes, whereas such duplications are uncommon in bacteria found in natural plants, animals, soil, and water.
“Most bacteria have some basic antibiotic resistance genes in them, but we rarely saw them being duplicated out in nature,” that’s what you said. “By contrast, we saw lots of duplication happening in humans and livestock where we’re likely hammering them with antibiotics.”
The researchers also found that the levels of resistance duplication were even higher in samples taken from clinical datasets where patients are likely taking antibiotics. This is an important point, they say, because the increase in copying antibiotic resistance genes also increases the likelihood of bacteria evolving resistance to new types of treatments.
“Constantly creating copies of genes for resistance to penicillin, for example, maybe the first step toward being able to break down a new kind of drug,” Maddamsetti said. “It gives evolution more rolls of the dice to find a special mutation.”
“Everyone recognizes there is a growing antibiotic resistance crisis, and the knee-jerk reaction is to develop new antibiotics,” you wrote. “But what we find time and again is that, if we can figure out how to use antibiotics more efficiently and effectively, we can potentially address this crisis much more effectively than simply developing new drugs.”
“The majority of antibiotics used in the United States are not used on patients, they’re used in agriculture,” you pointed out. “So this is an especially important message for the livestock industry, which is a major driver of why antibiotic resistance is always out there and becoming more serious.”