An acid is a substance that can donate a hydrogen ion (H+) to another substance. More specifically, it is a measure of the number of protons or hydrogen ions that are present in an aqueous solution. Acids have a pH of less than 7.0. Most acids contain a hydrogen atom bonded that can release (dissociate) to yield a cation and anion in water. A chemical can donate a proton if the hydrogen atom is attached to an electronegative atom like oxygen, nitrogen, or chlorine. Their chemistry makes them one of the most important classes of molecules in nature and science.
Acids may be identified as either strong or weak acids based on how completely they dissociate into their ions in water. The weak acids hold on to some of their protons, while the strong acids let go of all of them. All acids will release hydrogen ions into solutions. The number of ions that get released per molecule will determine if the acid is weak or strong. Weak acids are acids that partially release the hydrogen atoms that are attached. These acids, then, may lower pH by dissociation of hydrogen ions, but not completely. Weak acids generally have a pH value of 4-6 while strong acids have a pH value of 1 to 3.
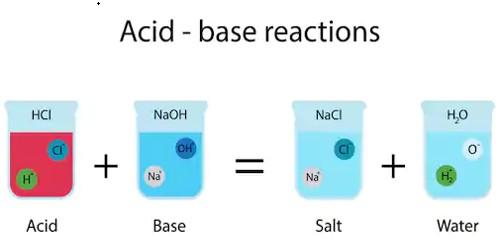
A base is an acid’s “chemical opposite.” A base is a substance that will accept the acid’s hydrogen atom. Bases are molecules that can split apart in water and release hydroxide ions. They are sour, sharp, or biting to the taste.
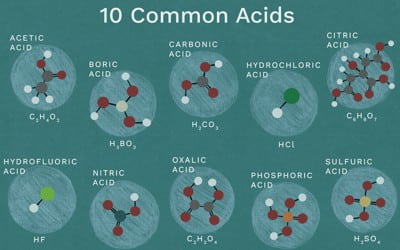
Acid Examples: Arrhenius acid, Hydrochloric acid, Sulfuric acid, Hydrofluoric acid, Acetic acid, etc.
How acids work
he pH scale is a scale that is used to represent the level of acidity in a solution. Acids and bases typically exist together in equilibrium. This means that within a sample of an acid, some molecules will give up their protons and others will accept them. Even water is a mixture of an acidic ion, H3O+ (called a hydronium ion) and a basic ion, OH– (called a hydroxide ion). An acid dissociates, or breaks apart, and donates protons, or hydrogen ions, in an aqueous solution, while a base donates hydroxide ions in a solution. This reaction happens continuously in a sample of water, but overall the sample is neutral because there are equal amounts of hydronium and hydroxide in the sample. For most reactions, however, the acids and bases are not present in equal amounts, and this imbalance is what allows a chemical reaction to occur.
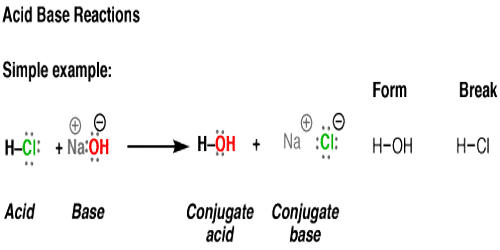
Every acid has a conjugate base formed by removing the acid’s proton. Hydrochloric acid (HCl), for example, is acid and its conjugate base is a chlorine anion or Cl–. Acid and its conjugate base are opposite in strength. Since HCl is a strong acid, Cl– is a weak base.
Properties
Acids can have a lot of different properties depending on their molecular structure. Most acids have the following properties:
- taste sour when they are eaten
- can sting the skin when they are touched
- can be used as a reactant during electrolysis due to the presence of mobile ions
- turn blue litmus paper red
- turn red or orange on a universal indicator
- conduct electricity
Acids can burn the skin, the severity of the burn depending on the type and concentration of the acid. These chemical burns require immediate medical attention.
Information Source: