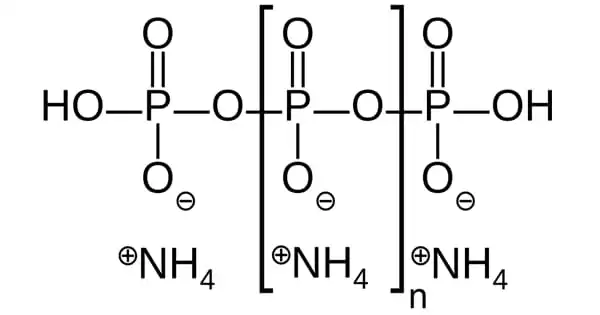
Ammonium polyphosphate is an inorganic salt of polyphosphoric acid and ammonia that contains both chains as well as the possibility of branching. Its chemical formula is [NH4 PO3]n(OH)2, indicating that each monomer is composed of an orthophosphate radical of a phosphorus atom with three oxygens and one negative charge neutralized by an ammonium cation, leaving two bonds available for polymerization. Some monomers in the branching situations lack the ammonium anion and instead bond to three additional monomers.
Ammonium polyphosphate is a fertilizer, emulsifier, and food additive. It is utilized in chemical synthesis as a compound cyclizing agent and an acylating agent. It is also used as an orthophosphoric acid replacement and as an analytical reagent.
Properties
The properties of ammonium polyphosphate are determined by the amount of monomers in each molecule and, to some extent, by the frequency with which it branches. Shorter chains (n<100) are more water sensitive and less thermally stable than longer chains (n>1000), yet short polymer chains (e.g., pyro-, tripoly-, and tetrapoly-) are more soluble and demonstrate increased solubility as chain length increases.
- Molar mass: 97.01 g/mol
- Appearance: white powder
- Density: 1,9 g/cm3; bulk density = 0,7 g/cm3

Preparation
Ammonium polyphosphate is made by combining concentrated phosphoric acid and ammonia. Iron and aluminum impurities, which are soluble in concentrated phosphoric acid, create gelatinous precipitates or “sludges” in ammonium polyphosphate at pH levels ranging from 5 to 7. Copper, chromium, magnesium, and zinc are examples of metal impurities that create granular precipitates. Ammonium polyphosphate, on the other hand, can operate as a chelating agent to maintain certain metal ions dissolved in the solution, depending on the degree of polymerization.
The emission of non-flammable carbon dioxide in the gas phase helps to dilute the oxygen in the air and the combustible decomposition products of the burning substance. In the condensed phase, the resulting carbonaceous char protects the underlying polymer from oxygen and radiant heat assault. When mixed with starch-based compounds such as pentaerythritol and melamine as expanding agents, it can be used as an intumescent. A series of publications describe the processes of intumescence and the method of action of APP.
Applications
Ammonium polyphosphate (APP) is utilized as a flame retardant in a range of applications, including paints and coatings, as well as polymers, the most important of which are polyolefins, particularly polypropylene, where APP is part of intumescent systems. The process of combining APP-based flame retardants with polypropylene is detailed in. Thermosets, where APP is employed in unsaturated polyesters and gel coatings (APP blends with synergists), epoxies, and polyurethane castings are further applications (intumescent systems). APP is also used to make flame-retardant polyurethane foams.
Long chains and a specific crystallinity characterize ammonium polyphosphates, which are utilized as flame retardants in polymers (Form II). At 240 °C, they begin to break down, producing ammonia and phosphoric acid. In the dehydration of carbon-based poly-alcohols such as cellulose in wood, phosphoric acid functions as an acid catalyst. Heat-unstable phosphate esters are formed when phosphoric acid interacts with alcohol groups. The esters break down, producing carbon dioxide and regenerating the phosphoric acid catalyst.