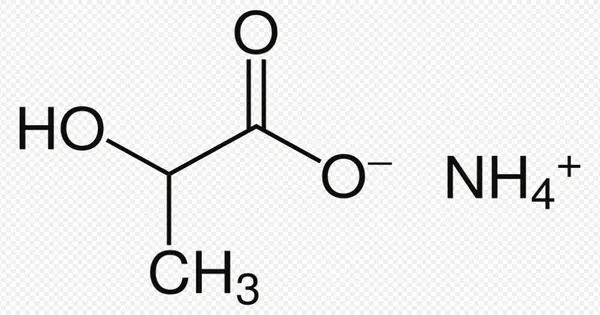
Ammonium lactate is a chemical having the formula NH4(C2H4(OH)COO). It’s the ammonium salt of lactic acid. It has modest antibacterial effects. It is a chemical produced by the interaction of lactic acid and ammonium hydroxide. Its qualities make it useful in a wide range of applications.
Ammonium lactate is a chemical compound composed of lactic acid and ammonium hydroxide. It is used to hydrate dry, scaly, and irritated skin. Those who use it should avoid direct sunlight and artificial UV rays, such as sunlamps or tanning beds. Ammonium lactate makes the skin more sensitive to sunlight, increasing the likelihood of sunburn.
Ammonium lactate is sold as an over-the-counter medication Amlactin brand used for treating xerosis.
Properties
- Appearance: Typically a colorless to light yellow liquid.
- Solubility: Highly soluble in water.
- pH: Usually slightly acidic to neutral, depending on the concentration.
- Stability: Generally stable under normal conditions but may be sensitive to high temperatures or strong acids/bases.
Applications
- Exfoliant: Ammonium lactate is commonly used in dermatological preparations as a gentle exfoliant. It helps to remove dead skin cells and improve skin texture.
- Moisturizer: It has moisturizing properties that can help treat dry skin conditions such as eczema or psoriasis.
- Lactic Acid Source: It provides lactic acid, which is known for its ability to promote cell turnover and improve skin appearance.
- Preservative: It can act as a preservative in some food products, although its use is less common compared to other preservatives.
- pH Adjuster: It can be used to adjust the pH of food products.
- pH Balancer: Used in pharmaceutical formulations to adjust pH levels and improve the stability of the product.