According to one study, amyloid-beta found in the blood is secreted by peripheral tissues (pancreas, adipose tissue, skeletal muscle, liver, and so on) that are responsive to glucose and insulin. In a new study, Osaka City University posits a probable mechanism linking diabetes to Alzheimer’s disease: amyloid-β in the blood comes from peripheral organs other than the brain, such as the pancreas and liver, and aids in blood glucose clearance by suppressing insulin secretion.
According to one study, amyloid-β (Aβ) found in blood is secreted by peripheral tissues (pancreas, adipose tissue, skeletal muscle, liver, and so on) that are responsive to glucose and insulin. Furthermore, Aβ released from peripheral tissues functions as a regulator on pancreatic β-cells, suppressing insulin secretion. According to the findings of this study, blood Aβ levels vary dramatically with food, and additional precautions should be followed when using blood samples as a diagnostic marker for Alzheimer’s disease, such as taking blood samples when fasting.
Researchers discovered that amyloid-beta (Aβ) seen in blood comes from peripheral organs and that the peptide acts on pancreaticβ cells to limit insulin release, hence regulating blood glucose levels. The study, which warns against using blood Aβ levels as a diagnostic marker for Alzheimer’s disease (AD), was published in The Proceedings of the National Academy of Sciences (PNAS), the National Academy of Sciences’ official publication.
“This work was eventually published after nearly 11 years,” says Professor Takami Tomiyama of Osaka City University Graduate School of Medicine’s Department of Translational Neuroscience. “It is not merely an intellectual discovery, but it also has consequences for how we detect Alzheimer’s disease.”
Our findings suggest that Aβ and organokines are stored during periods of fast and released into circulation when stimulated with insulin. A comprehensive understanding of the organokine action of peripheral Aβ is something we hope to develop in future work.
Prof. Tomiyama
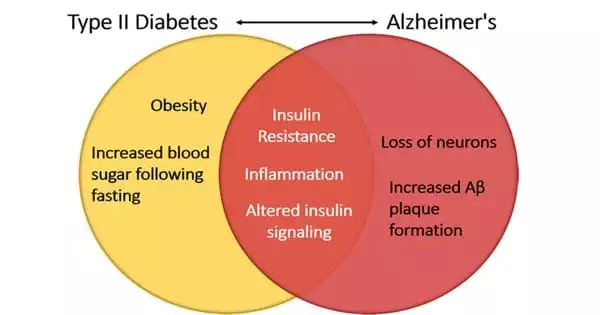
This study aimed to investigate several unknowns based on what was known. First, because Alzheimer’s disease is characterized by an accumulation of Aβ in the brain, Aβ levels in the blood are assumed to mirror the pathology in the brain and are currently utilized as a diagnostic marker. However, A is produced from the amyloid precursor protein (APP) by the action of two enzymes, β- and γ-secretases, and this mechanism is expressed in many of the body’s peripheral organs, not just the brain, leaving the origin of blood Aβ uncertain. Second, epidemiological studies have revealed that type 2 diabetes is a strong risk factor for the development of Alzheimer’s disease, but the mechanism linking these two diseases has also baffled experts.
“In prior experiments on mice injected with glucose, we found a transient increase in glucose and insulin to peak at 15 minutes, but blood Aβ levels to peak 30-120 minutes later,” Professor Tomiyama explains. Furthermore, earlier research has indicated that oral glucose treatment raises blood Aβ levels in Alzheimer’s disease patients. These findings prompted the professor and his research team to investigate the concept that blood Aβ is produced by peripheral tissues (pancreas, adipose tissue, skeletal muscle, liver, and so on) and may contribute to glucose and insulin metabolism.
First, they looked at the impact of glucose and insulin on blood Aβ levels in mice that had been fasting for 16 hours. Blood samples collected from the tail vein at 0, 15, 30, 45, 60, 120, and 180 minutes after the injection revealed a transient increase in glucose, insulin, and A, corroborating prior research.
They then investigated the effect of Aβ on blood insulin levels by giving A and glucose to fasting mice that are unable to make Aβ, known as APP knock out mice. When insulin levels in blood samples were measured over time, it was shown that Aβ inhibited the glucose-stimulated rise in insulin.
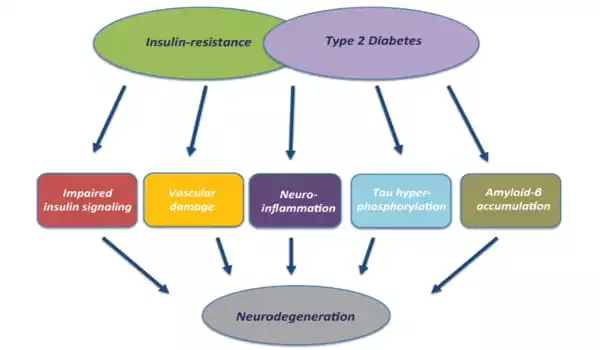
Given that blood Aβ levels changed promptly after the addition of glucose and insulin, the researchers concentrated on the pancreas, adipose tissue, skeletal muscle, liver, and kidneys of mice to determine the source of blood Aβ. They examined the amount of Aβ secreted after adding glucose and insulin to isolated live peripheral tissues. Aβ was secreted from the pancreas in response to glucose stimulation and from adipose tissue, skeletal muscle, and the liver in response to insulin stimulation. The kidneys, which are not involved in the metabolism of glucose or insulin, did not release Aβ in response to either stimulus. They also discovered that when glucose and Aβ were introduced to pancreas tissue, released insulin levels were decreased.
Now that the origin of blood Aβ had been clarified, the team wanted to localize Aβ in the periphery tissues studied. “This would elucidate the cells involved with Aβ,” adds Professor Tomiyama. “In addition to providing further validation to our findings, this would give us a more detailed picture from which we could draw conclusions to possible mechanisms connecting type 2 diabetes and AD.”
Using immunohistochemistry to take advantage of the fact that antibodies bind to specific proteins, the team began with pancreas tissue, finding Aβ only in insulin (β cells). The researchers also discovered that cells from mice given glucose had fewer immunoreactions to A and insulin, implying that during periods of fasting, Aβ and insulin are stored in cells and then released into circulation when stimulated with glucose. Similarly, tissue sections of each insulin-targeted organ were produced and immunostained for Aβ and organokines, which are bioactive molecules specific to each tissue. Aβ was discovered with all of the organokines examined, with reduced immunoreactions when induced with insulin.
“Our findings suggest that Aβ and organokines are stored during periods of fast and released into circulation when stimulated with insulin,” adds Prof. Tomiyama. “A comprehensive understanding of the organokine action of peripheral Aβ is something we hope to develop in future work.”
In addition to an explanation for the origin of Aβ in the blood, the research findings suggest a mechanism by which type 2 diabetes is a strong risk factor for the development of AD. In diabetes, Aβ levels in the blood are constantly elevated due to high levels of glucose and insulin. This inhibits Aβ to leave the brain to the periphery (transport through the blood-brain barrier and by body fluid flow through the brain parenchyma called the glymphatic system), causing Aβ to accumulate in the brain and develop into AD.
“Other more practical suggestions can be gleaned from this study,” concludes Prof. Tomiyama, “our data suggest that as blood Aβ levels fluctuate significantly with diet, special care should be taken when diagnosing AD with blood Aβ.”