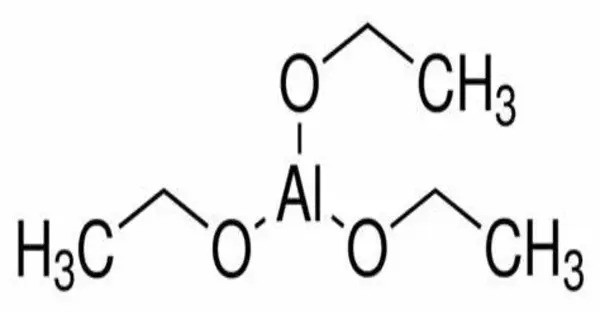
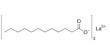
Aluminium ethoxide (also aluminium triethoxide) is an metallo-organic compound with the empirical formula Al(OCH2CH3)3. It is a chemical compound that consists of aluminium and ethoxide (C₂H₅O) groups. It is a versatile organoaluminum compound with significant use in catalysis, material science, and organic synthesis, although it is not found naturally in the environment and must be synthesized in controlled conditions. It is often used in chemical synthesis and industrial applications. It is a moisture-sensitive white powder.
Properties
- Chemical formula: Al(OCH2CH3)3
- Molar mass: 162.165 g·mol−1
- Appearance: White powder
- Density: 1.142 g/cm3
- Melting point: 140 °C (284 °F; 413 K)
- Boiling point: 320 °C (608 °F; 593 K)
- Solubility in water: reacts violently
- Solubility: slightly soluble in xylene, chlorobenzene
Properties
Aluminium triethoxide is slightly soluble in hot dimethyl benzene, chlorobenzene and other high boiling point non-polar solvents. It hydrolyzes to aluminium hydroxide and ethanol:
Al(OEt)3 + 3 H2O → Al(OH)3 + 3 EtOH
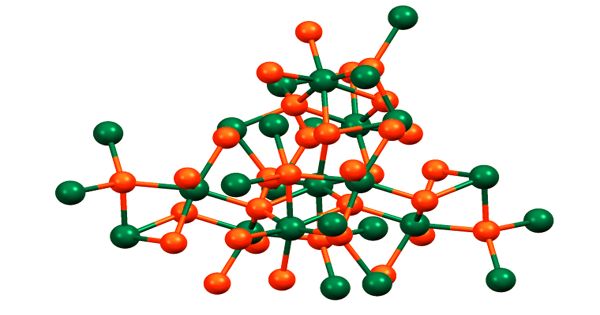
Although the structure of aluminium triethoxide has not been established by X-ray crystallography, the related aluminium isopropoxide has a tetrameric structure as verified by NMR spectroscopy and X-ray crystallography. The species is described by the formula Al[(μ-O-i-Pr)2Al(O-i-Pr)2]3. The unique central Al is octahedral, and three other Al centers adopt tetrahedral geometry.
Reactions
Synthesis: Aluminium ethoxide is often used as a precursor to other aluminium compounds, including aluminium oxide (Al₂O₃) and aluminium-based catalysts.
Catalysis: It is used in certain catalytic processes, especially in the production of organic materials and in organic synthesis reactions.
Water Reactivity: It reacts with water to form aluminium hydroxide and ethanol:
Al(C2H5)3 + 3H2O → Al(OH)3+3C2H5OH
Precursor to Aluminium Oxide: When hydrolyzed, it can produce aluminium oxide, which is useful in various industrial processes.
Applications
Aluminium triethoxide is used as a reducing agent for aldehydes and ketones, and is also used as a polymerization catalyst. Aluminium triethoxide is mainly used in Sol-Gel Process preparation of high purity aluminium sesquioxide, which is a polymerization agent. At the same time, it is used as a reducing reagent, for example, carbonyl compounds that restore to alcohol.
Safety and Handling
- Due to its reactivity with water, it should be stored in airtight containers and handled with care.
- Protective equipment such as gloves and goggles should be worn during handling to avoid skin and eye contact.
- It is also advisable to handle it in a well-ventilated area to prevent inhalation of any fumes released during reactions.