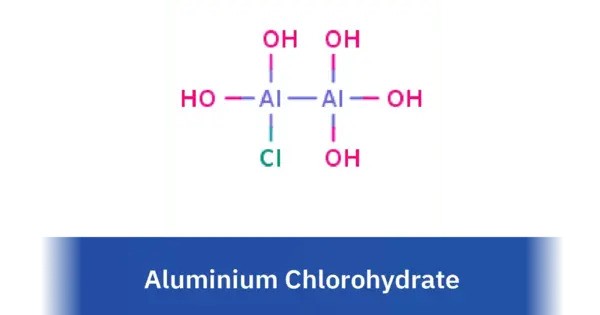
Aluminium chlorohydrate is a group of water-soluble, specific aluminium salts having the general formula AlnCl3n−m(OH)m. It is used in cosmetics as an antiperspirant and as a coagulant in water purification. It is a group of salts commonly used in deodorants and antiperspirants. It is also used in water treatment processes due to its flocculating properties.
In water purification, this compound is preferred in some cases because of its high charge, which makes it more effective at destabilizing and removing suspended materials than other aluminium salts such as aluminium sulfate, aluminium chloride and various forms of polyaluminium chloride (PAC) and polyaluminium chlorosulfate, in which the aluminium structure results in a lower net charge than aluminium chlorohydrate. Further, the high degree of neutralization of the HCl results in minimal impact on treated water pH when compared to other aluminium and iron salts.
Properties
- Molecular weight: Varies based on composition; for typical ACH: ~174.45 g/mol
- Solubility: Completely soluble in water
- pH (1% solution): Around 4.0 – 5.0
- Appearance: Clear to slightly yellow liquid (in solution form) or white solid
- Stability: Stable under normal conditions; decomposes at high temperatures
- State: Usually available as a liquid (aqueous solution), but also in solid form
- Viscosity: Depends on concentration; typically low to moderate
- Density: ~1.33 g/cm³ (40–50% solution)
- Melting Point: Decomposes before melting
Uses
Aluminium chlorohydrate is one of the most common active ingredients in commercial antiperspirants. The variation most commonly used in deodorants and antiperspirants is Al2Cl(OH)5 (dialuminium chloride pentahydroxide). It is also used as a coagulant in water and wastewater treatment processes to remove dissolved organic matter and colloidal particles present in suspension.
Antiperspirants & Deodorants:
- Reduces sweat by forming a gel-like plug in sweat glands.
- Most effective in clinical-strength antiperspirants.
Water Treatment:
- Acts as a coagulant—binds to impurities and helps them settle out.
- Preferred over alum in some systems due to lower sludge volume and better performance over a wide pH range.
Cosmetic and Pharmaceutical Applications:
- Sometimes used in formulations requiring aluminum salts for their astringent or antimicrobial properties.
Safety and Health
Topical use (e.g., in deodorants) is generally considered safe by regulatory agencies like the FDA and Health Canada, but:
- Some studies and public concern exist over aluminum compounds and breast cancer, though evidence remains inconclusive.
- Not recommended for use on broken skin or immediately after shaving.
- Environmental impact: Can be toxic to aquatic life in large quantities.