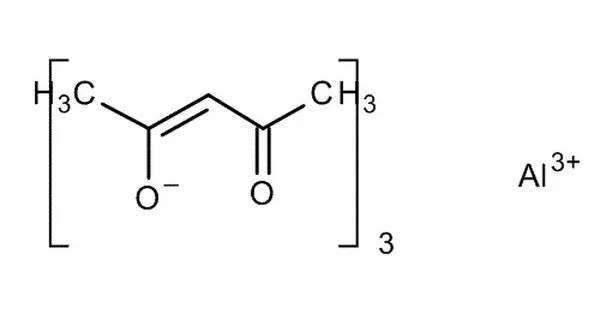
Aluminium acetylacetonate, also referred to as Al(acac)3, is a coordination complex with formula Al(C5H7O2)3. It forms a chelate complex where acetylacetonate ligands coordinate with the aluminium ion. This aluminium complex with three acetylacetone ligands is used in research on Al-containing materials. The molecule has D3 symmetry, being isomorphous with other octahedral tris(acetylacetonate)s. It is usually a yellow or orange powder that is soluble in organic solvents but not in water.
Properties
- Chemical formula: Al(C5H7O2)3
- Molar mass: 324.31 g/mol
- Appearance: White solid
- Density: 1.42 g/cm3
- Melting point: 190-193 °C
- Boiling point: 315 °C
- Solubility in water: Low
Uses
Aluminium acetylacetonate can be used as the precursor to crystalline aluminium oxide films using low-pressure metal organic chemical vapour deposition. In horticulture it can also be used as a molluscicide. Commonly used as a catalyst in organic synthesis, in the production of ceramic materials, and in the preparation of thin films. It also serves in applications involving metal-organic frameworks (MOFs).
- Catalysis: Utilized in various organic reactions to enhance reaction rates.
- Material Science: Employed in the synthesis of advanced materials, including ceramics and polymers.
- Thin Film Deposition: Used in processes like chemical vapor deposition (CVD) for producing thin films with specific properties.
Safety Considerations
While generally considered safe when handled properly, it’s advisable to use appropriate personal protective equipment (PPE) when working with chemical compounds to avoid inhalation or skin contact.