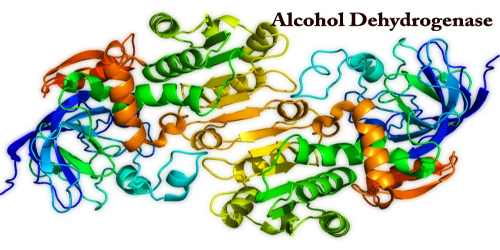
Alcohol dehydrogenases (ADH) are a group of dehydrogenase enzymes that occur in many species and promote the interconversion of alcohols and aldehydes or ketones to NADH by reducing the nicotinamide adenine dinucleotide (NAD+). The prefix ‘de’ means not, and ‘hydro’ refers to a hydrogen atom; so, alcohol dehydrogenase works by removing a hydrogen atom from alcohol. Some alcohol dehydrogenases catalyze the opposite reaction as part of fermentation in yeast, plants, and many bacteria, to ensure a steady supply of NAD+.
These help to break down alcohols that are otherwise harmful in humans and many other species, and they also participate in producing useful aldehyde, ketone, or alcohol groups during biosynthesis of different metabolites. While in our atmosphere there are several types of alcohol much of the research performed by alcohol dehydrogenase is on ethanol, the type of alcohol that we drink in beer, wine, and spirits. It is assumed that the ability to generate ethanol from sugar (which is the basis for how alcoholic drinks are made) originally originated in yeast.
ADH has five classes (I-V). Class 1 consists of α, β, and γ subunits that are encoded by the genes ADH1A, ADH1B, and ADH1C, and it’s used primarily in humans as a hepatic form. The human genes encoding class II, III, IV, and V ADH are ADH4, ADH5, ADH7, and ADH6, respectively. The activity level isn’t only addicted to the level of expression but also on allelic diversity among people. A study was conducted to find a correlation between alloy distribution and alcoholism, and the results suggest that the alloy distribution arose between 12,000 and 6,000 years ago, along with rice cultivation in the region. Rice was also fermented to ethanol in regions where rice was cultivated.
Alcohol dehydrogenase breaks alcohol all the way down to another toxic compound, acetaldehyde. Acetaldehyde may be a well-known toxin and a carcinogen, therefore, the body can’t keep this around; Excess acetaldehyde results in hangover symptoms like nausea, headache, malaise, sweating et al. The first-ever isolated alcohol dehydrogenase (ADH) was purified in 1937 from Saccharomyces cerevisiae (brewer’s yeast). The alcohol dehydrogenases comprise a gaggle of several isozymes that catalyze the oxidation of primary and secondary alcohols to aldehydes and ketones, respectively, and can also catalyze the reverse reaction.
Another enzyme called acetaldehyde dehydrogenase comes in to convert acetaldehyde to acetate, a harmless molecule that’s further weakened into water and carbon dioxide; these compounds can easily be released from the body. Alcohol dehydrogenase also leads to the toxicity of certain forms of alcohol: for example, methanol is oxidized to produce formaldehyde and ethylene glycol to eventually yield glycolic and oxalic acids.
Information Sources: